Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2019)Volume 10, Issue 1
Background: In the literature there are a few reported cases of cicatrical pemphigoid Brunsting-Perry type (CPBP). CPBP is well clinically characterized by clinical research, however is heterogeneous in a term of immunological findings. Laser scanning confocal microscopy (LSCM) is a helpful tool for diagnostics of cicatricial pemphigoid in general, especially when circulating antibodies are not detectable.
Objectives: Application of LSCM for the first time in a case with clinical features of CPBP and review the literature.
Methods: Skin biopsy was taken for LSCM studies. LSCM technique relies on comparison between the location of IgG/IgA deposits and basement membrane zone (BMZ) markers: antibodies directed to laminin 332 served as a marker of lower part of lamina lucida, whereas antibodies to type IV collagen served as a marker of lamina densa. Immunoglobulins are labeled with anti-human IgG-FITC (green staining), whereas BMZ markers are labeled with anti-mouse IgG-Cy 5 (red). Both fluorescence are simultaneously excited and overlayed by appropriate laser lines and asseses by computer program.
Results: LSCM studies disclosed both IgG and IgA located below laminin-332 and above type IV collagen, characteristic of cicatricial pemphigoid.
Brunsting-Perry CP; Mucous membrane pemphigoid (MMP); Epidermiolysis bullosa aquista (EBA); Laser scanning confocal microscopy; Dapsone
Original, Cicatricial Pemphigoid of Brunsting-Perry type (CPBP) was characterized by the development of recurrent vesicles and blisters with erosion formation, located on the scalp and upper part of the body which heal leaving atrophic scars [1,2]. The development of significant scars on the scalp leads to cicatricial alopecia [3]. Skin lesions are usually accompanied by chronic pruritus. A few cases of CPBP were reported with mucosal erosions or scarring conjunctivitis (Table 1). Currently, CPBP is considered to be a separate type of autoimmune blistering disorder with predilection to upper part of the body involvement.
| POS | REF. NO/SOURCE | SEX/AGE | LOCATION OF SKIN LESION | INCLUDE MUCOSE MEMBRANE | TREATMENT | D I F | I I F | Characteristic antigens (ELISA, IB) |
|---|---|---|---|---|---|---|---|---|
| 1 | Brusting (1957) 1 | M/55 | Right temple, scalp | N | CT, AH, SP | *N/A | *N/A | *N/A |
| 2 | Brusting (1957) 1 | M/70 | Left neck, trunk, arms | N | CT, AH, SP | *N/A | *N/A | *N/A |
| 3 | Brusting (1957) 1 | M/60 | Cheecks, forehead, left axilla, groin, chcest | N | HC, SP | *N/A | *N/A | *N/A |
| 4 | Brusting (1957) 1 | M/66 | Cheecks, neck, groins, hands | Y | PA, SP | *N/A | *N/A | *N/A |
| 5 | Brusting (1957) 1 | M/57 | Right forehead scalp, trunk | N | *N/A | *N/A | *N/A | *N/A |
| 6 | Brusting (1957) 1 | M/48 | Right jaw, forehead, neck, hands | N | *N/A | *N/A | *N/A | *N/A |
| 7 | Brusting (1957) 1 | F/40 | Right forehead, temple, chin | N | *N/A | *N/A | *N/A | *N/A |
| 8 | Jacoby (1978) 7 | M/50 | Right forehead, left side of neck | N | anti IgG, anti IgS, anti B,C/B,A | N/A | N/A | |
| 9 | Leenutaphong (1989) 8 | F/72 | Left preauricular region | N | TS, EM, D | linear deposits of IgG, C3 at dermal-epidermal junction | circulation IgG along BMZ | N/A |
| 10 | Joly (1993) 9 | M/84 | Left chick | N | D, P | IgG and C3 linear staining along basement membrane zone | negative | N/A |
| 11 | Poon (1999) 10 | M/59 | Vertex of the scalp | N | TS(B), D | negative | negative | N/A |
| 12 | Mitel (2000) 11 | M/74 | Over scalp, beard area, face, upper trunk | N | P, D, T | Linear IgG and C3 along BMZ | N/A | N/A |
| 13 | Mitel (2000) 11 | F/50 | Creases of neck, scalp | N | D | IgG along BMZ | N/A | N/A |
| 14 | Sugita (2001) 12 | F/33 | Face, neck | N | MC, NA, PS | Linear IgG and IgA AND along the BMZ | negative | N/A |
| 15 | Daito (2008) 13 | F/68 | Left scalp, upper arm, trunk | N | TS, DC, NA | linear IgG, IgA, IgM, C3 at the BMZ | circulating IgG at BMZ | IgG to C-terminal portion of BP180 |
| 16 | Martin (2009) 2 | F/63 | Vertex and occipital area of scalp | N | TS ( CP) | linear C3 at the BMZ | N/A | N/A |
| 17 | Tanaka (2009) 14 | F/65 | Face, neck, upper back | Y | P,D,C | Circulating IgG along BMZ | Collagen type VII collagen laminin-332 | |
| 18 | Takeichi (2009) 15 | M/56 | Forhead, bilateral cheeks, ear lobes | P | IgG and C3 linear staining along basement membrane zone | N/A | recombinant NC16a of BP180 was negative BP230, BP 180 positive | |
| 19 | Demitsu (2009) 16 | M/38 | Face, scalp, neck, upper trunk | N | D | IgG at epidermal BMZ, C3 deposits at the hair follicle BMZ | Circulating IgG along BMZ | recombinant NC16a of BP180 |
| 20 | Fukuda (2011) 17 | F/86 | Left preauricular region, left breast | N | TS | IgG deposition at BMZ, C3 deposition at the hair folicle BMZ |
IgG on both roof and floor of the SSS | Laminin 332, BP230, desmoplakins I/II |
| 21 | Jedlickova (2011) 18 | M/58 | Parietal scal region | Y | AB, AM, TS | weak linear IgG at the BMZ | negative | BP180 |
| 22 | Jedlickova (2011) 18 | M/74 | Top of the scalp | N | TS | negative | weak linear IgG, M and A along BMZ | laminin 332 |
| 23 | Minato (2011) 19 | M/31 | Chest, cheeks, neck, midchest | Y | P,NA,T | IgG and C3 along BMZ | IgG on the dermal side of SSS | collagen type VII |
| 24 | Garcia-Martin (2014) 20 | F/63 | Trunk, upper and lower limbs | Y | P, A | linear deposits of IgG and C3 along BMZ | IgG on the epidermal side of SSS | LAD-1 |
| 25 | Sato-Shibuya (2016) 21 | F/20 | Blisters on the face, neck, scalp | N | BTM | linear deposition of IgG antibodies along the BMZ and granular deposition of C3 to a part of the BMZ; no deposition of IgA or IgM was detected. | circulating IgG along BMZ | negative |
| 26 | Asfour (2017) 22 | F/46 | Face, cheeks, forhead and temples | N | D, P | linear IgG and C3 deposition along BMZ, localized on the dermal side on salt-split skin DIF-IgG, IgA along BMZ | circulating IgG along BMZ | negative |
| 27 | Present case | F/77 | Scalp, forehead, periauricular area, upper chest | N | P, D | LSCM-IgG located below laminin 332 and above collagen type IV | circulating IgG on the epidermal side in SSS | IgG to C-terminal portion of BP180 |
AB: antibiotics; AM: antimycotics; A: azathioprine; TS: topical steroid; B: beclomethason dipropionate; CP: clobetazol propionate cream; DC: doxycycline; T: tetracycline; C: colchicine; NA: nicotinic acid; P: prednisolone; NHS: normal human skin; BMZ: basement membrane zone; SSS: salt split skin.
Table 1: Twenty-six cases published as CPBP.
CPBP has some characteristic findings in histopathology, especially, subepithelial separation with or without leucocyte infiltration. Whereas in direct immunofluorescence the entity is characterized by in vivo bound IgG (and in some cases IgA) along the basement membrane zone (BMZ) in patients’ skin similarly to mucous membrane pemphigoid (MMP) and epidermolysis bullosa acquisita (EBA) [3]. By using immunelectron microscopy it was has been showed that IgG deposits are located on the border of lamina lucida and lamina densa [3]. Circulating antibodies, if detectable, recognize different BMZ antigens (BP180, collagen type VII, laminin 332), therefore the entity is heterogeneous [4].
We would like to present a case of Brunsting-Perry CP in whom for the first time the diagnosis was established using laser scanning confocal microscopy. Additionally, we analyzed all published cases with CPBP in a term of clinical, immunological and therapeutic aspects.
We present a case of a 77-year-old female, who come to the clinic due to extensive, well-defined, numerous blisters and erosions located on the forehead, upper chest and left preauricular region (Figure 1a). Additionally, the patient additionally complained on of a skin soreness and pruritus. No mucosal involvement was found. During anamnesis the patient had hypertension, diabetes mellitus type 2 treated with insulin and chronic kidney disease. She had been hospitalized 7 months before, for pneumonia with erythrocyte pleural effusion.
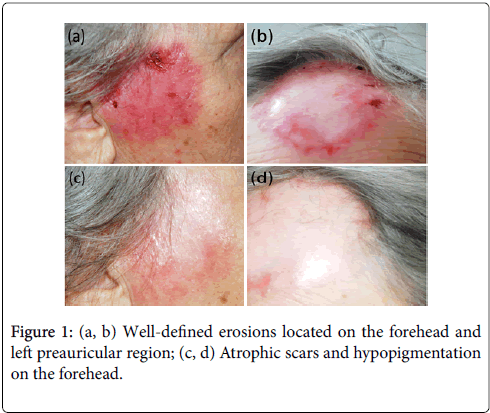
Figure 1: (a, b) Well-defined erosions located on the forehead and left preauricular region; (c, d) Atrophic scars and hypopigmentation on the forehead.
• Direct immunofluorescence (DIF) which has been performed during the hospitalization identified linear deposits of IgG and IgA along BMZ in patient’s skin (Figure 2a).
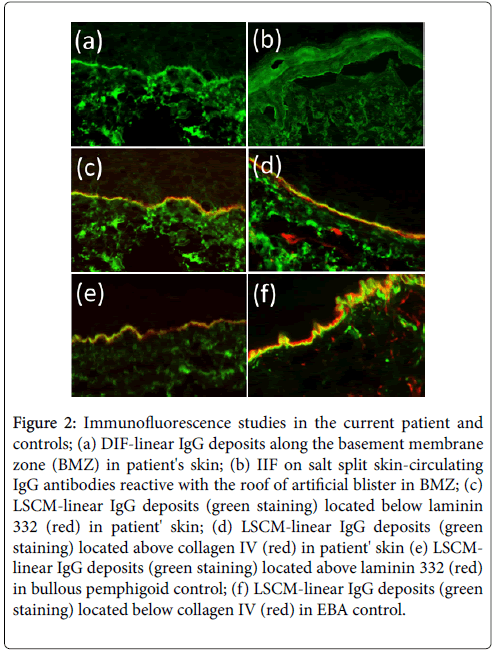
Figure 2: Immunofluorescence studies in the current patient and controls; (a) DIF-linear IgG deposits along the basement membrane zone (BMZ) in patient's skin; (b) IIF on salt split skin-circulating IgG antibodies reactive with the roof of artificial blister in BMZ; (c) LSCM-linear IgG deposits (green staining) located below laminin 332 (red) in patient' skin; (d) LSCM-linear IgG deposits (green staining) located above collagen IV (red) in patient' skin (e) LSCMlinear IgG deposits (green staining) located above laminin 332 (red) in bullous pemphigoid control; (f) LSCM-linear IgG deposits (green staining) located below collagen IV (red) in EBA control.
• Indirect immunofluorescence (IIF) which has been performed on a salt-split skin showed the linear staining of both IgG and IgA circulating antibodies with the floor of artificial blister at the titre 1:40 (Figure 2b).
• Immunoblot (IB) showed the reactivity of patient’s circulating antibodies with carboxyterminal end of BP180 BMZ antigen.
LSCM technique relies on comparison between the location of IgG/IgA deposits and BMZ markers: monoclonal antibodies directed to laminin 332 served as a marker of lower part of lamina lucida, whereas monoclonal antibodies to type IV collagen served as a marker of lamina densa. Immunoglobulins are labeled with anti-human IgGFITC (green staining), whereas BMZ markers are labeled with antimouse IgG-Cy5 (red staining). Both fluorescences are simultaneously excited and overlayed by appropriate laser lines and asseses by computer program [5-6].
LSCM study in the present case disclose both IgG and IgA located below laminin-332 and above type IV collagen, characteristic of cicatricial pemphigoid (nowadays named mucous membrane pemphigoid-MMP) (Figure 2c and 2d). In contrast, in bullous pemphigoid (BP) controls IgG were located above laminin 332, whereas in epidermolysis bullosa acquisita (EBA) controls IgG deposits were located below type IV collagen, as it has been showed previously (Figure 2e and 2f) [5-6]. The patient responded well to prednisone (20 mg/day) along with dapsone (50 mg/day). After a 2-month treatment, skin lesions healed leaving atrophic scars and hypopigmentation (Figure 1b). The pruritus relieved. During the follow-up period the patient remained free of symptom. The patient remained symptom free in the follow up period.
In 1957 Brunsting and Perry published seven patients with localized form of autoimmune blistering disorder. This patients presented tense blisters located mainly on the scalp and upper part of the body which were healing with atrophic scars. In one of them oral mucosa was also involved. These cases were published before the era of immunodermatology, therefore they were not characterized in a term of neither in vivo bound immunoglobulins nor target antigens [1].
During a few next decades there were published several cases with localized blisters diagnosed as a BP, EBA or MMP, and the proper Brunsting Perry CP [3]. Despite of the diagnosis localized autoimmune blistering disorders initially present very similar clinical features, clinically indistinguishable, however the course and prognosis may be varied [7]. In BP cases blisters and erosions heal leaving only hyperpigmentation only, without scars [5]. That patients usually require mild therapy but they have good prognosis. Whereas in MMP and EBA cases skin erosions clear with atrophic scars and milia formation and the diseases may have chronic and recurrent course with extension of skin and mucosal involvement [3]. These cases may require aggressive immunosupresive therapy to avoid complications, like esophagus stricture or scarring conjunctivitis [3]. Therefore, the differential diagnostics in cases with localized blisters are is mandatory. Routinely, for the diagnosis of BP it is required to show IgG deposits along the BMZ by DIF and reactivity of circulating IgG with NC16a domain of BP1808. In EBA cases circulating antibodies react with type VII collagen, whereas in MMP they are mainly directed to karbocsyterminal end of BP1803. However, circulating antibodies are rarely detectable in MMP and EBA cases (20% and 50% respectively) and the proper diagnosis is not possible [3,5].
In the literature there are only twenty-six cases published as CPBP (Table 1) [7-22]. This disease is common for middle-aged individuals, since where most of described cases were included patients in the age of 50-60. In the present case, ranks in 25% of the cases with 70-80 years old patients. It is worth noting that, patients younger than 50 years old represent group of 25% of all cases?
All of them had typical involvement of head and/or upper trunk, 6 out of 26 (23%) presented erosions on the extremities, and 5 out of 26 (19%) had oral erosions. This indicates that at least a few patients are in an analyzed group with incorrectly classified since they fulfilled clinical criteria of MMP [3]. DIF was negative in 3 (11%) cases and not performed in further seven cases (27%). On the basis of currently in force guidelines for BP, MMP and EBA positive DIF is mandatory for the diagnosis3. Circulating antibodies were detectable in only 10 out of 26 (38%) cases. The main target antigen was BP 180 (5 cases) characteristic of bullous pemphigoid, subsequently laminin 332 (3 cases), characteristic of ani-epiligrin cictarical pemphigoid, and carboxyterminal end of BP 180 (1 case) characteristic of MMP. Collagen type VII was the aim for circulating antibodies in 2 cases, this is typical for EBA.
In cases of autoimmune blistering disorders, in which circulating antibodies are not detectable, LSCM is crucial for the diagnosis of BP, EBA, MMP as we demonstrated previously [5,6]. In the current case there were showed as a first, IgG deposits located below laminin 332 and above collagen type IV, typically for anti-BP180-type cicatricial pemphigoid, thus we confirmed LSCM technique as a helpful tool. Moreover, the diagnosis obtained by LSCM was supported by IB results finally confirming the diagnosis of CPBP in the patient.
The current case received a typical treatment of CPBP-prednisone along with dapsone [3]. Among all analyzed cases, 33% patients also responded well to dapsone while 14% responded well to prednisone. Another 5 patients were treated with combination of prednisone and dapsone. The remaining patients were treated with topical corticosteroids or systemic antibiotics with success. Detailed data are summarized in Table 1. In general, CPBP is rather considered as a mild blistering disorder and usually require anti-inflammatory treatment only, however the analysis of previously described cases (Table 1) disclosed that most of that cases required more aggressive therapy. This suggests that they could have been rather cases of MMP or EBA than CPBP.
Since classification of blistering disorders is still a matter of controversy, more such cases may provide better understanding of relationship between clinical features and target antigens and subsequently therapeutic implications. In cases with no detectable circulating IgG anti-BMZ antibodies, LSCM has been shown to be crucial for the proper diagnosis.
Conflict of interest: None declared
Funding sources: The work was performed in the frame of the National Centre for Research and Development, Poland, STRATEGMED2/269807/NCBR/2015, Acronym: BIOOPA.
Author contributions:
• Study concept and design: Osipowicz, Wozniak, Jakubowska, Kowalewski.
• Acquisition of data: Osipowicz, Wozniak, Jakubowska, Kowalewski.
• Analysis and interpretation of data: Wozniak, Hashimoto, Kowalewski.
• Drafting of the manuscript: Osipowicz, Jakubowska.
• Critical revision of the manuscript for important intellectual content: Wozniak, Hashimoto, Kowalewski.
• Administrative, technical, and material support: Osipowicz
Citation:
Osipowicz K, Jakubowska B, Kowalewski C, Hashimoto T, Wozniak K (2019) Laser Scanning Confocal Microscopy for Diagnostics of Brunsting-Perry Type Cicatrical Pemphigoid Cases, along with Review of Literature. J Clin Exp Dermatol Res 10:479.
Received: 18-Nov-2018 Accepted: 18-Dec-2018 Published: 10-Jan-2019
Copyright:
© 2019 Osipowicz K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.