Journal of Fertilization: In Vitro - IVF-Worldwide, Reproductive Medicine, Genetics & Stem Cell Biol
Open Access
ISSN: 2375-4508
ISSN: 2375-4508
Research Article - (2019)Volume 7, Issue 1
Objective: To assess the safety of laparoscopic myomectomy and consider the underlying reason for the most serious complication; that of uterine rupture.
Design: 2 case studies with review of the recent literature.
Setting: Two Australian private facilities under a single medical directorship providing comprehensive services in gynaecology, andrology and reproductive endocrinology for infertility management including in vitro fertilization (IVF). Experience with advanced laparoscopic surgery over 35 years including myomectomy over 27 years.
Patients: Two women aged 37 years and 40 years respectively, who suffered uterine rupture post laparoscopic surgery to resect adenomyosis nodules, undertaken in order to improve their prognosis for IVF. Each woman now has a healthy child but one woman suffered an explosive uterine rupture which required emergency management at several levels. Both have been advised against further pregnancies.
Intervention: Applying the same laparoscopic techniques that work so well for myomata, namely using intramural pitressin to minimise bleeding and a 2-layered suturing method to close the myometrial defect, nodules of focal adenomyosis were resected.
Main outcome measures: Haemorrhage requiring blood transfusion and uterine rupture in ensuing pregnancy.
Results: Whilst no surgical complications occurred in 1600 cases of laparoscopic myomectomy, 2 cases of excess bleeding and 2 cases of uterine rupture in the ensuing pregnancy occurred in 200 case of adenomyosis resection.
Conclusion: Advanced diagnostic tests should be applied to differentiate those cases which have underlying adenomyosis and hormonal therapy may be preferred over surgical approaches for this enigmatic condition.
Laparoscopic myomectomy; Adenomyosis; Uterine rupture; Fibroids; Obstetric complications; Laparoscopic complications; IVF
This article presents an extensive clinical experience which highlights, on the one hand, the comparative safety of laparoscopic myomectomy along with the potentially more hazardous procedure of wedge resection of nodular adenomyosis. We include two dramatic case reports which highlight that although the laparoscopic operations appear to mostly go very well, the subsequent pregnancy outcomes can be extremely hazardous. The implication is that the surrounding myometrium contains diffuse adenomyosis which causes suboptimal healing of the myometrium.
The first laparoscopic myomectomy procedure conducted from our facility was 27 years ago (July, 1991) and since then more than 1600 procedures have been conducted by our group. A further 200 cases of laparoscopic “myomectomy” proved to be cases of adenomyosis following recognition during surgery and subsequent histological evaluation. PIVET Medical Centre is a tertiary facility managing all aspects of Infertility along with associated pathologies including endometriosis, fibroids & adenomyosis, tubal & pelvic pathologies as well as advanced laparoscopic surgeries for benign conditions with authors being rated to the highest level 6 as categorised by AGES; the Australian Gynaecological Endoscopy Society [1]. Two members have attained AGES 6-B (benign gynaecological surgery) and 6-R (reproductive gynaecological surgery); the other 2 have attained AGES level 5.
Cases of adenomyosis were sometimes obvious from the preoperative evaluation due to associated symptomatology of dysmenorrhoea, generalised pelvic pain and bloating and tenderness on pelvic examination. Others were suspected from ultrasound appearances, elevated CA125 levels and MRI studies although the latter was rarely used. Most cases were diagnosed at laparoscopy with the uterine irregularity proving to be non-discrete lumpiness without the clear dissection planes typical of benign myomata. Both singlemass adenomyomata as well as diffuse adenomyosis patterns were identified and our operative procedures have included myolysis as well as wedge resection of discrete and conglomerate adenomyosis masses.
Over the 27 year period we have conducted some “challenging” laparoscopic myomectomy procedures including the removal of 30 separate fibroids in one case (who has subsequently achieved 2 children spontaneously) and successful laparoscopic procedures completed where the uterus had sizes equating to 18-22 weeks gestation! It has been our long-standing practice to utilise intra-uterine pitressin (vasopressin; 20 units dissolved in 100 ml saline) at dosages limited to 4 IU (20 ml of diluted solution) per hour, and we can indicate that no case required blood replacement. However, we have now limited our cases to uterine size “16 weeks” in order to avoid long operative procedures as cases of post-operative atelectasis and thrombo-embolic conditions occurred in occasional cases when the operative time exceeded 3 hours; the patient positioned in steep Trendelenburg position for up to 5-hours in the most challenging cases.
At the beginning (1991-1995), myomectomy specimens were morcellated by laparoscopic scissors, a tedious and time-consuming process where scissors became unusable after each case. When the Storz electronic morcellator became available in Australia in 1995, we purchased the Rotocut device and continued to use it until current time. The device was a major advance reducing operating times markedly and the vast majority of cases were conducted under 2.5 h usually with the 12 mm but sometimes requiring the larger 15 mm rotor blade and we learnt to morcellate with minimised spinning and scatter of resected tissues.
A 36-year old woman G1P0 at 36+6 weeks gestation, experienced acute abdominal pain and collapsed in the toilet of a friend’s house. Her husband extricated her from the toilet with some difficulty as she was jammed against the door. Following a 7 km ambulance journey to the Albany Regional Hospital in Western Australia, an urgent laparotomy was performed revealing 3 litres of fresh blood in the peritoneal cavity. Her 2532 gram son was delivered by lower segment caesarean section with a low Apgar score of 2 and he required some resuscitation. Following his delivery the uterine fundus was delivered through the abdominal wound and revealed an explosive 8 cm X 6 cm stellate full-thickness rupture in the right fundus (Figure 1a).
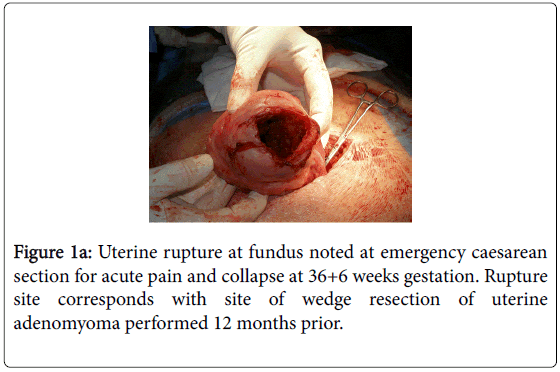
Figure 1a: Uterine rupture at fundus noted at emergency caesarean section for acute pain and collapse at 36+6 weeks gestation. Rupture site corresponds with site of wedge resection of uterine adenomyoma performed 12 months prior.
The infant was transferred by Royal Flying Doctor Service ambulance to the Tertiary Obstetric Facility in Perth, some 490 km distant. He was returned to his mother 4 days later in good clinical condition. His mother was estimated to have lost 3 litres of blood into the peritoneal cavity and was transfused 4 units of blood; she made an uneventful post-operative recovery.
The scarred area was excised and the fundal rupture sutured in a two-layer repair (Figure 1b).
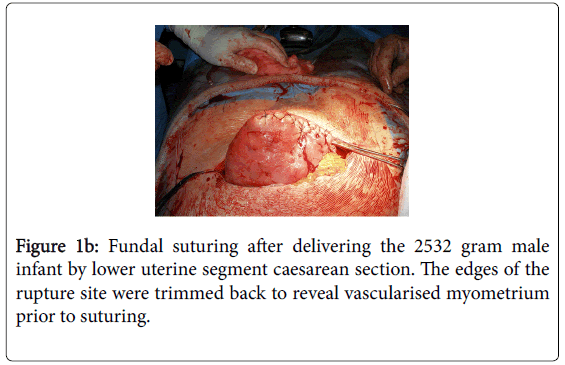
Figure 1b: Fundal suturing after delivering the 2532 gram male infant by lower uterine segment caesarean section. The edges of the rupture site were trimmed back to reveal vascularised myometrium prior to suturing.
Mother and child progressed well; the mother is using Implanon for contraception and has been advised against further pregnancies. Her son has progressed well with full cognitive skills. However, he was subsequently discovered to have a hemi-vertebra and is being managed by spinal brace. This congenital anomaly has no bearing on the maternal history of adenomyosis or the obstetric outcome.
It is undoubtedly relevant to note the woman’s previous history included 5 laparoscopic procedures for pelvic endometriosis causing long-standing dysmenorrhoea, dyspareunia and, subsequently, infertility. At PIVET, our main Fertility Centre in Perth, Western Australia, a further laparoscopic procedure was performed after 5 embryo transfers failed (One fresh following IVF/ICSI and subsequent 4 FETs). At this stage widespread adenomyosis was detected by ultrasound and the laparoscopy revealed a deeply retroverted uterus within a frozen pelvis comprising grade IV endometriosis invading the rectovaginal septum (Cullen’s disease). After block dissection of the endometriosis and ventro-suspension, a 4 cm uterine lump was also excised from the uterine fundus (Figure 2).
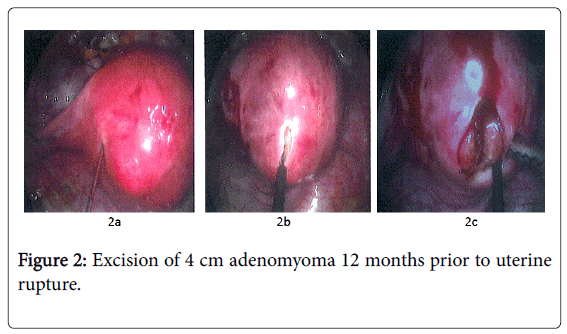
Figure 2: Excision of 4 cm adenomyoma 12 months prior to uterine rupture.
2a) Injection of 4 IU Pitressin adjacent to fundal adenomyoma for vasoconstriction to minimise peri-operative bleeding. 2b) Unipolar high-frequency electro cautery incision to open overlying myometrium revealing glandular intramural tissue rather than myoma which was initially expected. 2c) Highly vascular, glandular tissue excised by wedge resection as typical myoma plane absent. Although most of the adenomyomatous tissue was excised, lack of a clear plane and the associated diffuse adenomyosis meant some tissue inevitably remains in-situ and is incorporated in the deeper layer of suturing, limiting the healing process.
Zoladex implants 3.6 mg monthly were commenced and continued for the following 6 months. Further IVF procedures failed and another laparoscopy undertaken for myolysis to treat expanding adenomyosis. The myolysis procedure constituted 12 sites of high frequency electrocauterisation, avoiding the previous myomectomy site which however, was undetectable having a normal appearance, indicating it had apparently healed well from the laparoscopic suturing.
The pregnancy ensued following the transfer of two cryopreserved blastocyst-stage embryos under hormone replacement treatment (FET/HRT). The ET was conducted under trans-vesical ultrasound imaging and adjusted for the long cervical canal and bulky retroposed uterus. The clear fundal flash was detected at 9 cm from the external cervical os. Pregnancy was subsequently diagnosed and monitored through the first trimester as per PIVET protocol [2]. A normal singleton intra-uterine fetus with heart activity was demonstrated at 7 weeks and normal first trimester at 12 weeks. There after the patient was managed in her regional town Albany, being 490 km from Perth by road travel.
Second case report
A 40 year old woman with an IVF pregnancy achieved at our sister clinic Cairns Fertility Centre, in Queensland, was referred to a local obstetrician after the 7 week scan determined a live intra-uterine pregnancy. He reported back that the pregnancy became complicated by the development of gestational diabetes, requiring insulin therapy, and there was a major degree of placenta praevia. The patient had been hospitalised on two occasions for vaginal bleeding during her antenatal course. She was managed by elective caesarean section at 37+5 weeks gestation and a healthy female infant weighing 2990 grams was delivered with excellent Apgar scores. However, after removal of the placenta the obstetrician realized there was a defect in the posterior wall of the uterus. This was quite a large defect and was covered by peritoneum only (Figure 3a).
The obstetrician was aware of the previous laparoscopic surgery and surmised the defect was at the previous surgery site. There was no significant bleeding and he was able to undertake surgical repair. Mother and child had an uneventful recovery and the woman has been advised against trying for further pregnancies (Figure 3b).
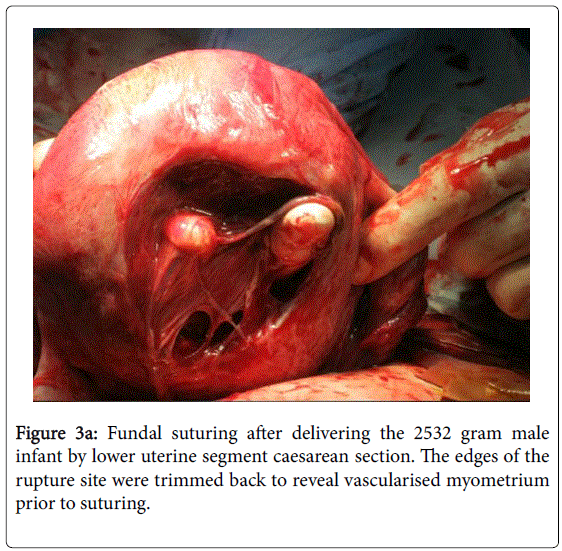
Figure 3a: Fundal suturing after delivering the 2532 gram male infant by lower uterine segment caesarean section. The edges of the rupture site were trimmed back to reveal vascularised myometrium prior to suturing.
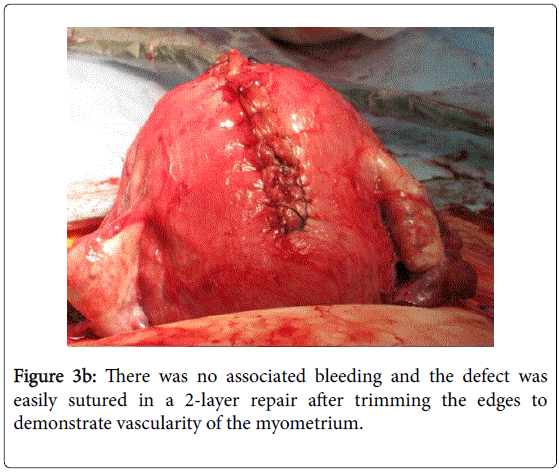
Figure 3b: There was no associated bleeding and the defect was easily sutured in a 2-layer repair after trimming the edges to demonstrate vascularity of the myometrium.
The laparoscopic surgery had been performed 12 months prior following two failed IVF procedures. Preliminary hysteroscopy revealed a normal sub-arcuate uterine cavity with some compression effect and adenomyosis at the postero-fundal region (Figure 4).
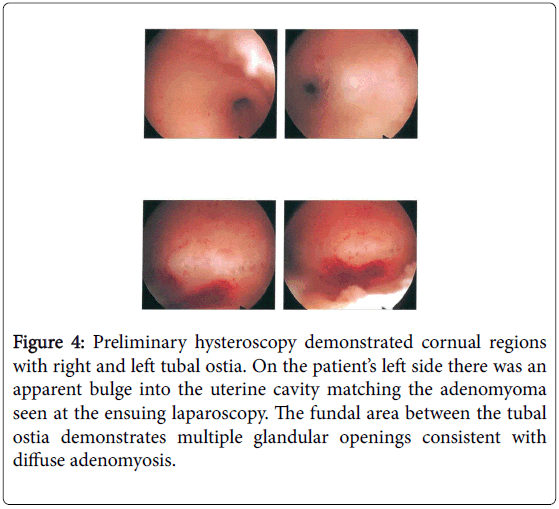
Figure 4: Preliminary hysteroscopy demonstrated cornual regions with right and left tubal ostia. On the patient’s left side there was an apparent bulge into the uterine cavity matching the adenomyoma seen at the ensuing laparoscopy. The fundal area between the tubal ostia demonstrates multiple glandular openings consistent with diffuse adenomyosis.
Laparoscopy revealed a 5 cm intramural “fibroid” which was excised under intra-mural pitressin cover (Figure 5).
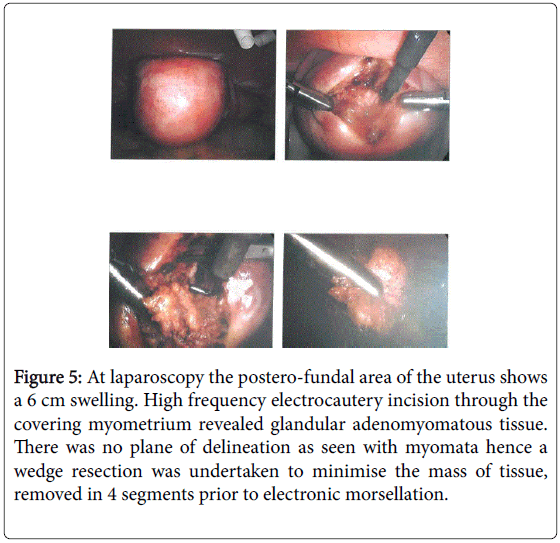
Figure 5: At laparoscopy the postero-fundal area of the uterus shows a 6 cm swelling. High frequency electrocautery incision through the covering myometrium revealed glandular adenomyomatous tissue. There was no plane of delineation as seen with myomata hence a wedge resection was undertaken to minimise the mass of tissue, removed in 4 segments prior to electronic morsellation.
During the dissection it became obvious that the nodule was not a fibroid as there was no distinct plane of dissection, and the excision became a wedge resection. None-the-less the cavity was closed quite neatly in a 2 layered repair using a continuous Quill barbed suture (Figure 6). Areas of endometriosis at the uterosacral crus were ablated and an incidental appendicectomy was also performed as the appendix was noted to be nodular and surrounded by adhesions. A RediVac drain was placed to the cul-de-sac. However, there was no bleeding during this day-case operation and post-operative recovery was entirely uneventful. Subsequent histology revealed normal proliferative endometrium, the nodule was classical adenomyosis with no malignant features and the appendix revealed extensive endometriosis with no malignant features.
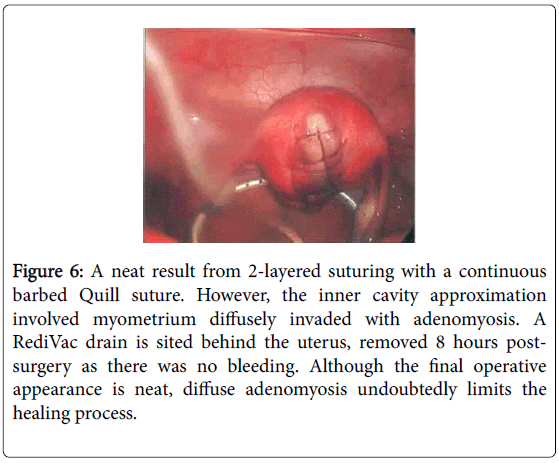
Figure 6: A neat result from 2-layered suturing with a continuous barbed Quill suture. However, the inner cavity approximation involved myometrium diffusely invaded with adenomyosis. A RediVac drain is sited behind the uterus, removed 8 hours postsurgery as there was no bleeding. Although the final operative appearance is neat, diffuse adenomyosis undoubtedly limits the healing process.
Two months later IVF was repeated and 2 cleavage-stage embryos (Day 3) were transferred fresh under ultrasound control. The uterus was noted to be ante-verted and was only mildly bulky. A clear fundal flash was seen at transfer indicating a well-sited procedure. Pregnancy was diagnosed 2 weeks later and ultrasound confirmed a singleton live fetus at 7 weeks (5 weeks post ET).
As noted, our experience with more than 1600 laparoscopic myomectomies indicated no operative complications, no cases requiring blood transfusion and no cases of uterine rupture in pregnancy (a formal audit of cases is in preparation). In cases or prolonged steep Trendelenberg position over 3 h, there may be increased risks for non-surgical complications such as atelectasis and thrombo-embolic phenomena which can be countered or avoided completely. On the other hand, the experience with laparoscopic wedge resection for adenomyosis and adenomyoma was vastly different in that serious complications were encountered in 4 cases among the 200 operations performed. These included 2 cases of uterine rupture in late pregnancy and one case of post-operative blood replacement (3-units) for post-operative bleeding via the routinely-placed Yates abdominal drain. A fourth case with 14-week uterus had laparotomy conversion as the adenomyoma proved impossible to mobilise from the uterine dissection – that case had emergency hysterectomy 8 hours later as massive secondary pelvic bleeding occurred from deep sacral vessels which had been excessively distracted (stretched) during the extraction process for the large, deeply embedded adenomyoma.
Our controlled morcellation technique, we have not experienced any cases of parasitic implantation and, although we discovered that 3 cases of our myomectomy specimens contained leiomyosarcoma at histology, none of these developed any metastases or displayed abdominal seeding and all 3 had successful management at the oncology service to which they were each referred.
The obstetric outcomes of post-myomectomy cases is the subject of a separate report, and can be summarised as no untoward events; however 2 of the cases which had wedge resection of nodular adenomyosis masses suffered uterine rupture during the ensuing pregnancies.
These 2 cases of uterine rupture ensued following excision of intramural nodules. One case was explosive, occurring in somewhat unfavourable, remote circumstances and the woman was lucky to have survived the event. The second case occurred silently and the woman appeared in no imminent danger as she was already resting up in hospital awaiting elective caesarean delivery for a major degree of placenta praevia. These 2 cases occurred 4 and 5 years ago respectively in circumstances where our laparoscopic expertise was well established over more than 20 years, but where we were extending the surgeries from “myomectomies” to wedge resection of adenomyosis nodules. Under pitressin cover, the surgeries appeared no more difficult than myomectomies, but it appears that the intramural tissue, being diffusely invaded by adenomyosis glandular tissue, may not have the same healing capacity as the compressed normal myometrium which surrounds myomata (fibroids). We have learnt to become more circumspect about diagnosing such cases by applying advances in ultrasound technology, by increased awareness and by the increasing use of magnetic resonance imaging (MRI) in uncertain cases. We have also become more reticent about conservative surgery for adenomyosis. Like its sister condition endometriosis, adenomyosis is an enigmatic condition which still challenges the fertility world regarding optimal management [3].
For cases desiring IVF, preliminary hormonal therapy including GnRHa agents combined with progestogens (we favour medroxyprogesterone acetate; Provera) should achieve reasonable control prior to conducting IVF treatment. Such cases may be best managed by cryopreservation of embryos whilst conducting several months of hormonal suppression. The use of ulipristal acetate was also an advanced consideration but unfortunately its potential for liver toxicity will restrict its use [4-6]. None-the-less we can expect further research utilising selective estrogen receptor modulators (SERMS) and progesterone modulators (SPRMS) will inevitably find safer alternatives. The idea of more complex surgeries requiring open laparotomy, providing an answer, is diminished by reports of uterine rupture occurring even after the overlapping procedures described by Osada [7].
However, against this problem described with adenomyosis, the laparoscopic myomectomy procedures appear quite safe and readily amenable to day-surgery [4,8,9]. We have not encountered any sort of surgical complication from over 1600 procedures, although we have now committed the very bulky cases to either sequential laparoscopies or a single laparotomy to reduce the time at which a patient is held in the steep Trendelenburg position required for Laparoscopic myomectomy on the more challenging cases. Fibroids do cause implantation problems as well as obstetric disorders hence an audit of pregnancies arising after laparoscopic myomectomies is currently underway from our series [10]. Our favourable experience has caused us to reduce the threshold for offering laparoscopic myomectomies in the IVF setting expanding beyond the generally accepted indication of Types 0, 1, 2 and 3 fibroids according to FIGO categories [11,12]. We believe that all intramural fibroids can interrupt the inner uterine vasculature, even those categorised 4 and 5 by FIGO as was eminently described by Sampson a century ago and which can cause adverse effects on implantation, even from locations in the outer myometrium, not just at the junctional zone, adjacent to the endometrium [13,14].
We conclude therefore that laparoscopic myomectomy should be offered readily for those cases of infertility proving resistant to standard treatments including IVF as well as those women suffering recurrent miscarriage. However, we would express caution regarding any conservative surgical management for adenomyosis and furthermore, diagnostic efforts should be undertaken to ensure that fibroids are indeed myomas, and not masquerading with underlying adenomyosis. The latter can be a cause of operative complications, especially haemorrhage, as well as serious obstetric complications, especially uterine rupture.
We conclude therefore that laparoscopic myomectomy should be offered readily for those cases of infertility proving resistant to standard treatments including IVF as well as those women suffering recurrent miscarriage. However, we would express caution regarding any conservative surgical management for adenomyosis and furthermore, diagnostic efforts should be undertaken to ensure that fibroids are indeed myomas, and not masquerading with underlying adenomyosis. The latter can be a cause of operative complications, especially haemorrhage, as well as serious obstetric complications, especially uterine rupture.
Grateful acknowledgement to the non-specialist obstetricians and anaesthetists at Albany Regional hospital who handled the emergency procedures for the first case and provided the pictures for Figures 1a and 1b. Grateful thanks also to Dr. Thomas G Wright, obstetrician at Cairns Private Hospital who managed the delivery of the second case and provided the pictures for Figures 3a and 3b. Thanks also to the heroic mothers who each approved release of their medical details.
Citation:
Yovich JL, Lingam S, Rowlands PK, Srinivasan S (2019) Laparoscopic Myomectomy Beware Adenomyosis. J Fertil In vitro IVF Worldw Reprod Med Genet Stem Cell Biol 6:212
Received: 01-Dec-2018 Accepted: 14-Jan-2019 Published: 21-Jan-2019
Copyright:
© 2019 Yovich JL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.