Medical Safety & Global Health
Open Access
ISSN: 2574-0407
ISSN: 2574-0407
Review Article - (2021)Volume 10, Issue 2
Safety principles are based on containment and risk assessment. The fundamentals of containment include the microbiological practices, safety equipment, and facility safeguards that protect laboratory workers, the environment, and the public from exposure to infectious microorganisms, chemical hazardous and radioactive exposure. In this article, we discussed the requirements and platform of biological research laboratories integrating safety issues as part of protection/hazard prevention and control program in the work environment in academic centers, including universities.
Laboratory research safety; Containment; Safety guidelines; Personal protection equipment
In the last decades, safety and biosafety gained increased concern during technical progress and development in life sciences, medical biotechnology in research laboratories in academic centers and pharma industries.
In academic labs, safety is considered a priority. The dynamics in which old labs, renew labs and novel labs are always in routine research work, lead the academic organization to build a consist Safety Laboratory Program and infrastructure to establish safety standards.
The scope of research laboratory safety support activities that may involve exposure to bio hazardous agents, hazardous chemicals, radionuclides and X-ray damages. The Academic Organization must offer an Institutional Safety Program that compiles all the categories [1]. The University is committed to providing a health and safe learning, teaching, research and work environment [2-7], Sackler Safety website: https://en-med.tau.ac.il/safety-on-fculty-ofmedicine2020.
Safety management
The advantage of an academic research lab compared to pharma industries is the “small scale” category in chemical use, biosafety agents and radionuclides. However, the diversity of all the categories is ample in the academic organization compared to the industry, reflecting the various research project fields [3,7]. In addition, academic safety managements are less stricken rules than pharma companies [8]. Reasonably, the academic platform has to provide tools to minimize accidents [9]. Assessment on laboratory safety knowledge at universities showed that students and researchers were rated with low score responses and deficiencies in emergency question [10,11].
For improvement of safety knowledge, Table 1 denotes the Basic Safety Program as well as Safety Guidelines.
| Safety Program Goal | University-wide Safety Guidelines and Infrastructure |
|---|---|
| Provide the resources necessary for research to be conducted in a manner that reduces the potential for exposure to infectious materials and prevent laboratory-acquired infections | Annual Safety Program to be sign by each research laboratory |
| Prevent environmental contamination | Safety website as a basic manual containing information and knowledge on principles and safety concepts |
| Secure experimental materials | Tutorials availability for the University staff and students on chemical, biosafety, radiation/X-ray and autoclave |
| Comply with State and local regulations | Recommendations for medical surveillance that may be required for staff, faculty and students |
| Safety committee in charge to review, discuss and approve safety issues, research projects and formulating policy for each safety item | Provide information on Personal Protection Equipment (PPE) |
| Apply sanctions on any individual whom violated the terms of safety guidelines | Sustain chemical and biological spill stations |
| Principal Investigator (PI) conduct risk assessment to identify potentially hazardous procedures involving infectious agents, instruct and train all staff and students working in the lab on safe work practices | Organize and provide chemical, biological and radioactive waste platform |
| Provide eyewash and shower public stations | Annual chemical monitor check for the chemical compounds most common use in biological labs as isoflurane, formalin, xylene |
| Principal Investigator (PI) to provide Personal Protection Equipment (PPE) for the lab members including protection for chemicals, biological agents and radioactive work practices | Annual regulation inspection of all radioactive rooms, X-ray/radioactive equipment |
| Revision on recombinant and synthetic nucleic acid molecules must be conducted as well as the use of bacterial and viral particles in recombinant vectors | Provide Safety Officers for each Faculty who acquainted lab experience and professional knowledge on safety issues: chemical, biology and radioactive |
Table 1: Denotes the safety program and safety guidelines.
Safety needs to maintain an existing lab or a new research lab is divided in several steps:
• The academic Safety Unit has to authorize a putative lab program/design according to the lab research needs, taking in consideration the risk factors
• Most biological research labs work is based on tissue culture, molecular technology and the use of chemicals as basis for the research
• Labs should include fume chemical hood and biological safety cabinets (BSC) with HEPA filter in order to prevent chemical gas inhalation as well as bioaerosol contamination droplets/particles
• For radionuclide research use and X-ray/Roentgen equipment, additional regulations are needed.
Laboratory safety training review by the principal investigator (pi) or designee
Principal Investigator is responsible to ensure that all personnel in the lab have the necessary skills (through training and experience), maturity and supervision to work safely in a lab with hazardous processes or substances. The PI may authorize another person to operationally fulfill the role. Consider the varying maturity and experience levels when orientating a person to the lab and when determining the appropriate assignments and supervision and training required [4].
Under PI supervision:
• Review the individual’s research program, identify core and specialized training requirements. Exception for those who will not be working with or adjacent to hazardous materials, processes or equipment
• Show researcher how to access training as tutorials in various fields and to acquainted the academic safety website
• Read laboratory-specific safety training/SOPs/Experiments protocols
• For highly hazardous materials, equipment, or processes that pertain to the individual’s research program (may include protocols, radiation registration, in addition to internal lab documents on carcinogens and cytotoxic reagents)
• Update the laboratory chemical inventories, the amounts increase or decrease, new chemicals are added, any chemicals which are no longer in use in the laboratory
• Prepare digital copies of Material Safety Data Sheet (MSDS) library in a computer lab station access to all laboratory workers
The following list is a basic safety feature in which the PI should guide his lab members:
• Lab emergency response guide and location of emergency numbers
• Emergency evacuation route and meeting area
• Location of fire extinguishers
• Location and proper use of safety showers and eyewash stations
• Location of Material Safety Data Sheets (MSDS) in lab or online (example Tel Aviv University, Sackler Faculty of Medicine https://en-med.tau.ac.il/safety-med-MSDS2020)
• Location of public building chemical and biological spill kits
• Accident report filling process
• Personal protective equipment (PPE) in the lab and PPE academic policy (gloves, safety glasses, goggles, lab coat)
• Vaccination policy: Hepatitis B and Tetanus
• Waste management: chemical, biological, sharp, cytotoxic and radioactive
• Location and proper use of chemical fume hoods and biosafety cabinets
• Location and proper use of chemical fume hoods and biosafety cabinets
Biosafety
The term biosafety is used to describe the procedures and policies adopted to ensure the environment and personal safety [12,13]. Laboratory biosafety practices are based on the principle of containment of biological agents to prevent exposure to laboratory workers and the outside environment.
Primary containment protects the laboratory workers and the immediate laboratory environment from exposure to biological agents.
Secondary containment protects the external laboratory environment from exposure to potentially hazardous agents of laboratory workers and environment.
The combination of primary PPE and secondary containments will reduce the exposure of the pathogens [14-16].
The main biosafety topics consist of the following:
• Laboratory design and maintenance: a lab’s arrangement and layout is there to make scientific research as smooth as possible and keep researchers comfortable improving productivity and minimizing errors
• Biological safety cabinets and other primary containment devices: good microbiological practice and procedure [17].
• Personal protective equipment (PPE): refers to clothing and equipment to protect the user from specific hazardous.
• Decontamination and waste management: cleaning, disinfection and sterilization.
• Biosafety program management: risk control strategy and standard operating procedures (SOP)
• Outbreak preparedness and resilience: early-warning surveillance, no work is risk free, prevents handling of leaking or badly packed specimens, operational procedures are in place and personnel involved in the work have been appropriately trained [18-22].
• Risk assessment: ways to reduce the risk are based on the use of micro-volumes, avoid culture and propagation of the pathogen, inactivate clinical specimens before disposal and analysis, and use non-infectious controls.
Hazardous chemicals
Chemicals liquids and powder are the essences of a research laboratory at the Academic system. The efforts in organizing chemicals in the Faculty are a key in minimizing accidents risks [23]. Chemical practices are divided in: storage, handling and moving. Data on particular reagents are found in compendia of reagents (https://www.rsc.org/Merck-Index).
The main hazardous chemicals topics consist as the following:
• Storage separation: minimize chemical storage. Hazardous chemicals should be separated due to their toxicity, flammability or reactivity
• Storage segregation: strong acids should be kept away from strong bases; highly-flammable liquids should be kept from all other materials, strong oxidizers away from organic or flammable materials
• Storage ventilation: toxic substances, especially high volatile, are stored within a cabinet that should be vented in a way that vapors, fumes escape from the close cabinet
• Handling: workers should handle chemical after training and become familiar with the MSDS. Preferable to handle small amounts of chemicals for research use and to open the original bottles in a fume hood. Keep chemical containers closed unless actively in use. High hygienic habits in the lab as removing personal protective equipment before leaving the lab, washing hands constantly, never eat, drink, smoke, apply cosmetics or contact lenses manipulation
• Moving: it is advisable to pack and move chemicals and hazardous materials during regular work hours. Prepare check list events including a spill or accident possibility. The University should provide public eyewash and shower stations in addition to emergency cabinets for a small spill event. Become familiar with all spill equipment, fire extinguishers and emergency exits.
Radionuclides and X-ray
An academic radiation tutorial/program presents the information necessary for the users of radioactive materials and radiation producing machines to properly understand and follow the University policies and procedures [24,25]. Occupational medical examination/consultation is conducted for the radioactive workers under dosimeter monitor. The topics to cover are: safe use of laboratory equipment and radionuclides, safe handling, storage and disposal of radionuclides, protective clothing, and methods to control and measure the radiation levels, radiation body doses and putative contamination.
The main radionuclides/X-ray exposure topics consist as the following:
• The basic safety standards prescribe permissible levels of exposure to radiation and fundamental operational principles creating “the code of practice”: Time, distance, and shielding and contamination control. Minimize exposure time; maximize distance using forceps and tongs; use appropriate shielding according to the radioactive source (glass stock vial, plexiglass, over lead and steel).
• External dosimeter: Radiation dose monitor (whole body) is requested for the occupational radiation dose when a sort of radionuclides/X-ray machines are used. Thermo luminescent dosimeters (TLD) are supplied and processed by independent outside companies. TLD is a small crystal which absorbs the energy from radiation. When heated, the badge releases the stored energy in the form of visible light. It measures the whole body dose and shallow dose and consists of a TLD crystal and a holder. The holder has several filters which help in determining the type and energy of radiation. The badge will detect gamma and x-rays, high energy beta particles, and in certain special cases, neutrons. It does not register radiation from low energy beta emitters such as H-3 (tritium), C-14 (carbon-14), and S-35 (Sulphur-35), since their betas will not penetrate the plastic covering on the TLD holder
• Contamination control: Wipe test method (for H-3, C-14 or S-35); Geiger probes (direct reading method for high energy betas, X-rays and gamma radiation); combined both methods
• Disposals: All waste or decay storage area is part of the inventory. Radioisotopes should be disposed in solid waste containers. Advisable not to accumulate container radioactive waste, i.e., an external company should collect the waste routinely
Risk management and accidents
Results from several studies have suggested that researchers are disinclined to conduct safety assessment prior to conducting experiments [26-28].
The factors for risk assessment can be summarizing in two categories: agent hazardous (chemicals, bioagents, radionuclides) and laboratory procedure hazards.
Risk assessment should be reviewed after implementation of selected safeguards, proficiency of staff regarding safe practices in the lab and after complete the integrity of safety equipment. The value of the Safety Unit support is to overcome the competency gaps increasing the professional training for students, workers and PIs [28-30]. To update and adapt safety regulations for each lab is also fundamental to minimize the risks. In Figure 1, a refined safety lab program is denoted starting from a lab construction to an ongoing laboratory.
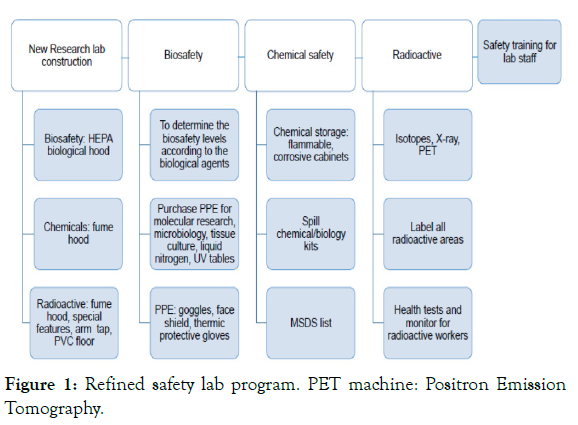
Figure 1: Refined safety lab program. PET machine: Positron Emission Tomography.
Altogether, there are still accidents in the labs [23,31].
In the event of gross chemical contamination on the body, the laboratory worker should remove contaminated clothing, activate the safety shower and stand under the water for a minimum of 15 minutes, then seek medical assistance. Behavior of the PI/Lab manager/Designee is the first step on the comprehension of the event and a reliable report including details like the chemical/ pathogen/radioactive source of the “spill”, spill place and dispose manner will be the base for future prevention contaminations.
One of the keys for keeping safety organization effectiveness is the routine inspection and investigation of any laboratory accident and reporting any fail in safety organization. Academic and safety lab organization must be keeping its preparedness in high level with regular exercises/simulation and reviewing its safety protocol effective and fulfilling any gaps (Figure 1).
Practical assessments should be conducted before the laboratory is operational to ensure that the selected risk control measures are feasible in the outbreak context.
Test runs and exercises mock specimens to demonstrate risk control measures. Test runs of equipment and facilities with performance check, exercise challenges with simulated hazardous spills and tracing contamination should be applied.
Human error is the main cause of accidents in the labs. Therefore, experienced, competent, well-trained and safety-conscious laboratory personnel are essential to lower the potential risk of chemicals, hazardous bioagents or radionuclides contaminations.
When properly handled, radiation sources represent a minimal risk to research personnel. In order to minimize the likelihood of accident events, an understanding of the principles of radiation protection is essential. Tutorials on radionuclide safety/ methodology and Safety Unit support will give a strong foundation in these principles as well as tools necessary to decontaminate and evaluate hazardous situations that may arise.
Whenever feasible, general laboratory ventilation, chemical fume hoods and secondary containments should be implementing to minimize exposure of hazardous chemicals.
Safety officers and Safety Unit at the Academic milieu is occasionally underdeveloped, necessary call and re-examination to encourage and implement safer lab practices will improve the lab environment safety.
The digital revolution, globalization of many academic institutes, new organizational structures, changes and introduction of novel technologies continue to transform the research work leading the need of a digitalized Safety Program System to fit each lab in the university. The digital safety program will provide an outcome that expresses the closest risk assessment score of the lab approaching the accident prevalence rate to a minimum.
Cordially grateful to Prof. Ilana Lotan for the support and revision of the article.
Citation: Rapaport D (2021) Laboratory Organization Integrating Safety on Chemicals, Bioagents and Radionuclides in Academia: A Systematic Review. Med Safe Glo Heal 10:153.
Received: 05-Oct-2021 Accepted: 19-Oct-2021 Published: 26-Oct-2021 , DOI: 10.35248/2574-0407.21.10.153
Copyright: © 2021 Rapaport D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.