Entomology, Ornithology & Herpetology: Current Research
Open Access
ISSN: 2161-0983
ISSN: 2161-0983
Research Article - (2020)Volume 9, Issue 4
Ectoparasites of cattle particularly tick and lice are global problems and even cause serious economic losses to the farmer and tanning industry in Africa particularly in Ethiopia. The objective of the present study was to evaluate the acaricidal properties of P. dodecandra (L. Herit), C. aurea (Ait) (Benth.) and R.communis (L) against adult A. variegatum (Fabricius) ticks. Leaves of three test plants were collected and prepared in extracts with concentrations of 150 mg/ ml, 100 mg/ml, 50 mg/ml, and 25 mg/ml using distilled water as a solvent for extraction. The experiment was arranged in CRD. Thirteen petridishs were prepared and in each petridishs 30 active adult A.variegatum ticks were added. Three plants extracts with different concentrations and diazinon 0.1% was used as standard positive control. After five minutes, immersed ticks were recovered and deaths of ticks were recorded after 1 hr, 6 hr, 12 hr, 24 hr and 48 hr intervals. Among the three selected plants C. aurea resulted in significantly higher mortality (p<0.05) against A. variegatum in different concentrations and exposure time. The 100% mortality was recorded by 100 mg/ml and 150 mg/ml and 90% and 96.6% mortality by 25 mg/ml and 50 mg/ml concentrations after 48 hours. P.dodecandra leaf extracts at 25 mg/ml concentrations had activity (46.66%) and 150 mg/ml concentrations (73.33%) after 48 hr exposure. Similarly, R.communis had moderate acaricidal activities, (73% and 80%) at lower concentrations and (96% and 100%) with higher concentrations respectively after 48 hr exposure. Based on the results obtained it can be concluded that the application of P.dodecandra, C.aurea and R.communis leaf extracts had important efficacy against A. variegatum cattle ticks.
Acaricide; Amblyomma variegatum; Concentrations; Mortality; Plant extract
The cattle industry plays an important role in the agricultural sector and the global economy of the world. Cattle production contributes about 25%-30% of the total agricultural output per annum [1]. Ethiopia is currently considered the tenth largest livestock producer and biggest exporter of livestock in Africa (UNECA, 2012) [2]. Parasitic diseases mainly ectoparasites are global problems and pose serious economic losses to the farmer, and the tanning industry of the country as a whole [3]. Tick and tick borne disease affect 90% of the world’s cattle population and are widely distributed throughout the globe. In eastern Africa, tick borne diseases are a concern to livestock farming leading to massive losses of livestock due to deaths [4].
As a result, several methods to control ticks have been identified and to date the most effective tick control method is site specific and repeated acaricide applications. However, a number of problems, including multi chemical resistance by pest organisms, cost of application, poisoning of treated animals and humans, resiudes in meat and milk and, environmental contamination especially in water bodies, are associated with their usage [5].
Ethno veterinary acaricides offer resource poor farmers an alternative to synthetic acaricides because they are cheap, familiar to the locals, being degraded in the environment, do not remain in livestock, are not as prone to resistance, and are relatively safe for humans, animals, the environment, they have also greater accessibility with lower costs and apparent effectiveness [6]. In a botanical survey conducted in Eastern Shewa, Ethiopia many plants were taken as the dominant medicinal plants used by healers to control insect pests and other ectoparasites [7].
However, limited research works are conducted in Ethiopia, especially in Machakil district of East Gojjam Zone of Amhara region to evaluate selected plant extracts against A. variegatum on cattle. There is a knowledge gap in the use of appropriate dosage and identifying better efficacious plant extracts against different species of cattle tick in the study area. Therefore, this study was conducted to evaluate the efficacy of extracts selected from botanicals against A. variegatum (Fabricius) ticks under laboratory conditions in the study area.
Description of the study area
Experimental study was conducted from January 2019 to May 2020 in Amanuael preparatory school, biology laboratory room, in Machakil district, which is one of the 19 districts of East Gojjam zone, Amhara regional state of Ethiopia located in 100 22'30" N to 100 44'30" N latitude and 370 20'30" E to 370 42'30" E longitude (Figure 1). It is also found at a distance of 300 KM North West of Addis and 270 KM south of Bahir Dar, the regional center and 30 Km from Debre Markos, the capital of East Gojjam zone. The total area of the study district is about 79,556 hectar [8]. Subsistence agriculture together with livestock breeding and trade are the dominant economic activities of the district. Land and livestock are the most important livelihood assets of the study area. Also in the district there are about 25 governmental veterinary institutions, seven private veterinary clinics and 12 veterinary drug centers [9].
Figure 1: Map of the study area.
Experimental study design
The experiment was arranged in a Complete Randomized Design (CRD) with three replications. The number of treatment was 13 which were composed from combination of three types of botanicals (P.dodecandra, C. aurea and R. communis) leaf extracts with four concentrations (25, 50, 100 and 150 mg/ml) and 0.1% Diazinon as standard check.
Selection of plant material
Plant material selection was carried out based on interviews and questionnaires served for local farmers to specify the indigenous plant species known for their use to control ectoparasites in their localities and through analysis of the relevant literature. A field survey was conducted to identify traditional medical practitioners and medicinal plant vendors with background knowledge on plants. The data about medicinal plants collected from ten resource farmers depended on traditional healings experience and skills. Each herbalist identified and provided three ethnoveterinary plants, which had anti tick activities based on their life experience. Out of these plants, 10 which are frequently listed by resource farmers were used for selection of test plants for the study. From the 10 plants, three with the highest frequencies using independent reports were selected.
Plant collection and identification
Study plants were collected from their natural habitats around Machakil district from November to December 2019. Leaves of three study plants, P. dodecandra, C. aurea, and R. communis were collected on the basis of reports on their traditional uses against various ectoparasites [10]. During collection, latex gloves were worn to reduce possible contamination, especially by fungi. Identification was carried out at Debre Markos University by expertise and identified plants were confirmed and voucher specimens were deposited at the University, Department of Biology laboratory class.
Plant preparation and aqueous extraction
The collected plant leaves were washed with distilled water to remove dirt and soil particles and cut into small pieces, spread out on paper sheets, and dried under a shaded area at room temperature for two weeks and finally crushed into fine powder using mortar and pestle. Crushed plant powder was labeled and the labeled plant powders were kept in plastic bags for a few days in the refrigerator until use. Plant extraction was conducted at Amanuael preparatory school biology laboratory room. To prepare the plant extraction, 25 g, 50 g, 100 g and 150 g powder from each plant was measured and put in a separated plastic bottle. Then, each measured powder was dissolved with the same amount of 1000 ml of distilled water and shake regularly for 10 minutes. Later the solution was filtered using a Whatman filter paper (No. 1: 125 mm) and the filtrate was stored in capped and labeled plastic bottles at optimum temperature until the experiment was conducted.
Tick collection, transportation and identification
Removal and collection of A. variegatum was carried out in May 2020. Primarily with the help of farmers, cattle which were not sprayed chemical acaricide in the season were identified. Then from naturally infested cattle a total of 390 similar sized adult ticks were collected from preferably attached body sites; lower dewlap, brisket, axilla, groin, udder and scrotum after proper physical restricting, casted or lay down of the animals excluding pregnant cows. During collection quality forceps, latex gloves and blunt tweezers were used to completely pull ticks off the animal and spray was used for sterilizing the area to prevent sepsis and possibilities of opportunistic infection on the animal [11].
Freshly collected ticks were placed in perforated plastic containers with freshly cut grass for providing moisture. Species identification and sex differentiation of ticks were done using the pictorial guide provided by Walker et al. [12]. Engorged females were not collected for this study as their larger size as compared to males produces data that does not follow the probit model when males are included in the analysis [13]. To solve this problem, other researchers tend to use larvae of uniform known age to evaluate response of ticks to toxicants. However, for plant-based acaricides to be useful, they must show significant acaricidal activity against adult ticks, as this stage is harder to kill and is also the initiator of tick control in resource poor smallholder cattle farming systems. Collected ticks were identified according to morphological identification keys given by Walker after preserved by 70% ethanol. Morphological features such as eyes, mouthpart, enamel ornamentation, color of legs, color of shields, marginal spots were used [12].
Adult mortality test
The experiment was carried out in three plant aqueous extracts with concentrations of (150 mg/ml, 100 mg/ml, 50 mg/ml and 25 mg/ml), per treatments and control petridishs [14]. Thirty active adult ticks were counted and randomly placed in thirteen Petri dishes [15]. Plant extract with different concentrations and 0.1% standardized diazinon was added to each petridishs until all containing ticks were completely immersed [16] and left for five minutes of exposure. After five minutes of contact time, the ticks were recovered from immersion, filtered with filter paper and placed in separate Petri dishes [17]. The botanical experiment was done in five observation times 1 hr, 6 hr, 12 hr, 24 hr, and 48 hr after treatment application. The viability of ticks was checked regularly by stimulation with a needle and ticks were considered as dead if no reaction was shown. At each replication time, dead ticks were counted, recorded and removed. The percentage mortality was calculated by the formula previously used by Krishnaveni and Venkatalakshmi [18] as follows.
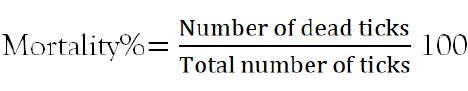
Acaricidal effect of selected plant was classified as follows; Very high when mortality was >90%; high when mortality was 70%-90%; moderate, when mortality was 50%-70%; weak, when mortality was 30%-50%; no or little, when mortality was <15% [19].
Data analysis
To examine the effect of the treatments on percentage mortality, a three-way ANOVA, generalized linear model was used. SPSS version 20 computer program was used for the analysis. Mean values were separated using the Tukey student test (HSD) procedures. Significance levels were given for p<0.05. The dose response estimation of the LC50, LC90, and the 95% confidence intervals of upper confidence limit and lower confidence limit were analyzed by probit analysis.
Acaricidal activity of P. dodecandra (L. Herit) against A. variegatum (Fabricius)
Mortalities of A. variegatum treated with different concentrations of P. dodecandra leaf extract were carried out in the study. Before 6 hour post exposure, almost all concentrations of the extract had no activity on tick mortality (<15%). At 24 hr post exposure, 100 mg/ml and 150 mg/ml concentrations of the extract had weak mortality of 33.33% and 44.16% and 25 mg/ml and 50 mg/ml concentrations showed little activity 14.16% and 18.33% in tick mortality respectively (Figure 2).
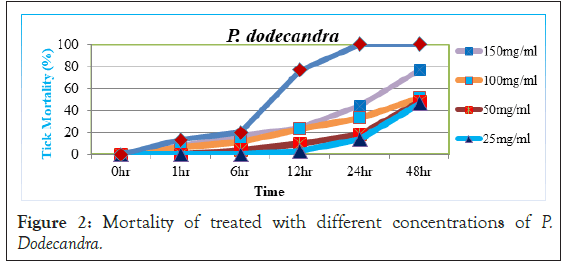
Figure 2: Mortality of treated with different concentrations of P. Dodecandra.
But, significant increase in tick mortality was started after 48 hour post exposure period, since 25, 50, 100 and 150 mg/ml concentrations of P. dodecandra has caused from moderate to high mortality (46.66%, 47.76%, 51.11% and 76.66%) respectively. Also, when the acaricidal activity at lower concentrations of P.dodecandra extracts were compared with other botanicals and diazinon, both concentrations had not significantly (p>0.05) higher in activity implying that lower concentrations of P.dodecandra had little acaricidal effect after 48 hrs. Still, 100 and 150 mg/ml of the extract had moderate efficacy with percentage mortality of (51.11%-76.66%) after 48 hr exposure. This study indicated that P. dodecandra was not as effective as the remaining extracts and standard diazinon. Inline this, the report of Namulindwa et al. [20] which showed aqueous leaf extract of P. dodecandra as moderately toxic towards Rh. decoloratus.
Acaricidal activity of C. aurea (Ait) Benth against A. variegatum (Fabricius)
As Figure 3 portrays, little tick mortality was started before 6 hour post exposure treated with 150 mg/ml concentrations of the extract. At 12 hr post exposure, 25 mg/ml and 50 mg/ml concentrations of the extract show mortality of (11.67% and 15%), while 100 mg/ ml and 150 mg/ml concentrations had mortality of 31.66% and 50% respectively. However, significant increase in tick mortality was observed at 24 hr with the last three higher concentrations (44.43%, 70% and 80%) and 48 hr post exposure 90% with 25 mg/ ml of C. aurea extract. Inline to the present study, acetone extracts of C. aurea would have acaricidal activity since [21] reported that 20% and 10% acetone extracts for C. aurea (leaves) either kill or severely compromise the mobility of adult A. variegatum ticks. It was suggested that extracts from C. aurea can potentially be used as baits in a trap for the control of ticks in the field [22]. Other researchers also revealed that C. aurea leaves and other products were shown to promise insecticidal activities against different insect and other arthropod pests. Adebayo et al. [23] reported that C. aurea used to kill lice and leaf extracts were applied in southern Ethiopia for protecting livestock against ticks. This is because it contains active compounds which are called quinolizidine alkaloid calpurnine 12 β, 13 α dihyidroxylic acid Easter. These quinolizidine alkaloids are toxic to insects and animals. Similar activities were found in C. aurea collected from Ethiopia and South Africa growing under widely different environmental conditions [24].
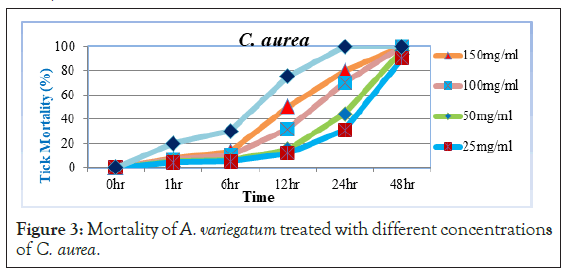
Figure 3: Mortality of A. variegatum treated with different concentrations of C. aurea.
Acaricidal activity of R. communis L. against A. variegatum (Fabricius)
Before 12 hour post exposure, little or no tick mortality was observed with 25 mg/ml and 50 mg/ml concentrations (5.88% and 13.33%), while 100 mg/ml and 150 mg/ml concentrations had weak mortality (26.66% and 31.66%) respectively. However, significant increase in tick mortality started 24 hour post exposure with 150 mg/ml and 100 mg/ml concentrations (66.66% and 50%) of the extract respectively. After 48 hour post exposure even 25 mg/ml and 50 mg/ml of the extract showed higher mortalities (73.33% and 80%) (Figure 4). The extract had a very high percentage mortality of 96.66% and 100% with two higher concentration and long time exposure. Even at lower concentrations R. communis was showed significant mortality (73.33 and 80%) after 48 hours. It has been reported that the high toxicity of R. communis was due to the presence of Ricin in the extracts [25] which is one of the most poisonous natural compounds [26,27]. Moreover, previous phytochemical studies reported that the crude leaves extract of the plant was positive for saponins, flavonoids, glycosides, fixed oils and fats, while negative for alkaloids, carbohydrate, tannins and phenolic compound and proteins and amino acids [28].
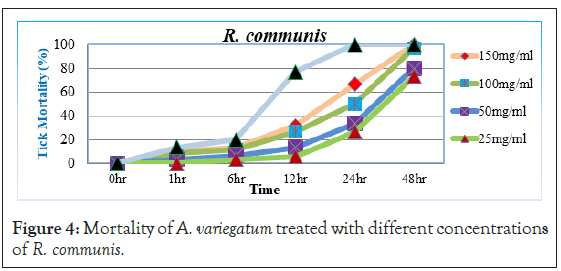
Figure 4: Mortality of A. variegatum treated with different concentrations of R. communis.
Comparative efficacy of aqueous extracts
Results indicated that at 48 hr leaf extract of C. aurea (90%, 96.66%, 100%and 100%), R. communis (73.33%, 80%, 96.66% and 100%), and P. dodecandra (46.66%, 47.76%, 51.11% and 76.66%) mortality with 25 mg/ml, 50 mg/ml, 100 mg/ml and 150 mg/ml concentrations respectively and there was 100% mortality from standardized diazinon (Figure 5). Hence, the two higher concentrations of C. aurea and only the higher concentration of R. communis had significantly (p<0.05) higher efficacy like diazinon at 48 hour post exposure. At the same time P. dodecandra had significantly (p<0.05) moderate efficacy only with higher concentration (150 mg/ml).
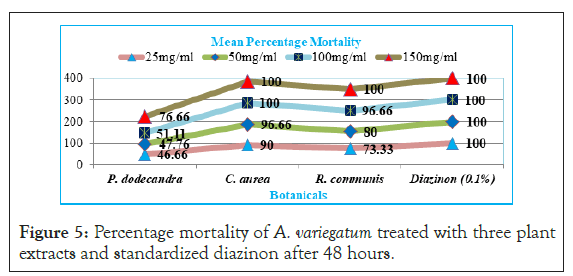
Figure 5: Percentage mortality of A. variegatum treated with three plant extracts and standardized diazinon after 48 hours.
The result showed that among the tested botanicals, aqueous extract of C. aurea (leaf) at higher concentrations had high percentage mortality (100%) towards A. variegatum after 48 hours, and this was similar to that of the standard diazinon 0.1% which caused 100% mortality after 24 hr.
The results obtained in the present study indicated that P. dodecandra and R. communis had lower acaricidal efficacy on A.variegatum at lower concentrations and short time exposure. In agreement to the present finding, Ghosh et al. [29] have reported that similar methanolic extract of the plant had potent activity on Rh. decoloratus even at lower concentrations. Absence of efficacy at lower concentration in the present study may be attributed to differences in species of parasite or differences in extracting solvent which the extracts were tested. It could also be related to the nature of the plant at the time of collection [30].
Mean mortality of A. variegatum due to different concentrations of botanical extracts
The result showed that the higher concentrations of these treatments caused significantly (P ≤ 0.05) higher mortality against A. variegatum only after 48 hours exposure time. Since, C. aurea stands first; it caused strong acaricidal activity like diazinon whereas R. communis had moderate acaricidal activity and P. dodecandra had the least based on their order of relative efficacy after 48 hours exposure (Table 1). The reason might be due to the bioactive botanical plant extract not uniformly present in every concentration and exposure time.
| Botanicals | % Mortality (M+SE) after treatment time | |||||
|---|---|---|---|---|---|---|
| Concentration (mg/ml) | 1hr | 6 hours | 12hours | 24 hours | 48 hours | |
| P. dodecandra | 25 | 0.00 ± 0.00b | 0.00 ± 0.32b | 2.23 ± 0.31kl | 14.43 ± 0.30l | 46.66 ± 0.00no |
| 50 | 0.00 ± 0.00b | 4.43 ± 0.03c | 8.90 ± 0.31j | 18.90 ± 0.59n | 47.76 ± 0.33o | |
| 100 | 6.66 ± 0.00e | 16.66 ± 0.00ij | 23.33 ± 0.00bc | 33.33 ± 0.00cde | 51.10 ± 0.32op | |
| 150 | 7.76 ± 0.31f | 16.66 ± 0.00ij | 24.43 ± 0.31jk | 44.16 ± 0.31jkl | 76.66 ±1.31hij | |
| C. aurea | 25 | 4.43 ± 0.31c | 5.56 ± 0.31d | 11.10 ± 0.31h | 31.10 ± 0.30def | 90.00 ± 0.00ac |
| 50 | 5.56 ± 0.30d | 6.66 ± 0.00e | 14.43 ± 0.30l | 44.43 ± 0.30jkl | 96.60 ± 0.55abc | |
| 100 | 7.66 ± 0.32g | 10.00 ± 0.56de | 31.10 ± 0.29def | 70.00 ± 0.00gh | 100.00.00a | |
| 150 | 11.10 ± 0.30h | 15.56 ± 0.31hi | 50.00 ± 0.52m | 80.00 ± 0.53fg | 100.0 ± 0.0a | |
| R. communis | 25 | 0.00 ± 0.00b | 3.33 ± 0.00i | 6.66 ± 0.50e | 26.66 ± 0.56lm | 73.33 ± 0.57q |
| 50 | 3.33 ± 0.00i | 6.66 ± 0.00e | 13.33 ± 0.55f | 33.33 ± 0.50cde | 80.00 ± 0.56fg | |
| 100 | 6.66 ± 0.00e | 11.10 ± 0.31h | 26.66 ± 0.51g | 50.00 ± 0.55m | 96.66 ± 0.54ab | |
| 150 | 8.90 ± 0.33j | 13.33 ± 0.00f | 32.33 ± 0.61cd | 63.33 ± 0.55efg | 100.0 ± 0.0a | |
| Diazinon | 0.10% | 13.33 ± 0.00k | 23.33 ± 0.54bc | 76.66 ± 0.55hij | 100.0 ± 0.0a | 100.0 ± 0.0a |
Mean within the same column and raw followed by the same letters are not significantly different, P>0.05%, Tukey student test (HSD).
Table 1: Mean mortality (± SE) of A. variegatum due to different concentrations of three selected plant extracts.
This means that higher concentration and longer exposure period are required to achieve applicable management of A. variegatum. After 48 hours exposure treated by 150 mg/ml of C. aurea, R. communis and P. dodecandra, adult tick mortality was 100%, 100% and 76.66% respectively.
In the present study the effect of tick mortality at different exposure time intervals showed a different scenario. Before 24 hour exposure time, all the three plant aqueous extracts exhibited little acaricidal activity and had not significant (p>0.05) mortality except 0.1% diazinon towards A. variegatum (Figure 6). However, at 48 hour, except P. dodecandra the remaining two plants aqueous extracts started to show significant (p<0.05) mortality aginst A. variegatum.
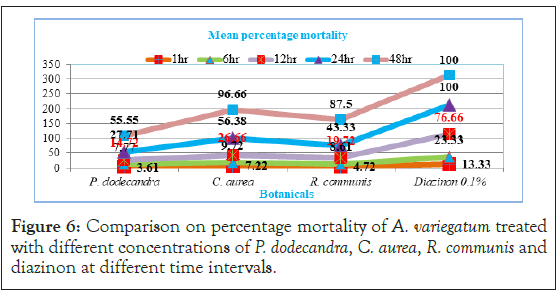
Figure 6: Comparison on percentage mortality of A. variegatum treated with different concentrations of P. dodecandra, C. aurea, R. communis and diazinon at different time intervals.
LC50 and LC90 of aqueous extracts of botanicals against A. variegatum
The probit analysis, estimation of LC50 and LC90 and 95% confidence intervals with lower and upper confidence limit at 1, 6, 12, 24, and 48 hours after treatment application was examined. Among the three tested plants, the highest acaricidal activities were observed in C. aurea, which had LC50 and LC90 values of 2.93 and 3.37, at 1 hour and 0.89 and 1.41 at 48 hours respectively. Leaf extract of P. dodecandra had LC50 and LC90 values of 3.50 and 4.80 at 1 hour and 1.64 and 4.68 at 48 hours respectively. Similarly, R. communis had LC50 and LC90 values of 3.02 and 3.87 at 1 hour and 1.16 and 1.17 at 48 hours. The results clearly showed that the percentage of mortality is directly proportional to the concentrations of the extract exposure time (Table 2).
| Botanicals | After 1 hour | After 6 hours | After 12 hours | After 24 hours | After 48 hours | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LC50(LCL-UCL) | LC90 (LCL-UCL) | LC50 (LCL-UCL) | LC90 (LCL-UCL) | LC50 (LCL-UCL) | LC90 (LCL-UCL) | LC50 (LCL-UCL) | LC90 (LCL-UCL) | LC50 (LCL-UCL) | LC90 (LCL-UCL) | |
| P.dodecandra | 3.5(2.84-23.76) | 4.8(3.75-44.3) | 3.25(2.67-6.44) | 3.83(3.13-6.48) | 2.53(2.33-2.94) | 3.3(2.90-4.24) | 2.33(2.16-2.67) | 3.2(2.73-5.86) | 1.64(1.35-1.80) | 3.18(2.68-4.76) |
| C. aurea | 2.93(2.53-4.38) | 3.37(2.92-4.40) | 2.69(2.42-4.16) | 4.15(3.31-7.29) | 2.25(2.13-.45) | 3.03(2.74-3.60) | 1.71(1.62-1.79) | 2.44(2.29-2.69) | 0.89(0.06-1.14) | 1.41(1.17-1.52) |
| R. communis | 3.02(2.58-5.26) | 3.87(3.09-7.90) | 2.93(2.55-4.31) | 4.56(3.45-10.8) | 2.49(2.29-2.92) | 3.42(2.97-4.49) | 1.96(1.85-2.11) | 2.99(2.66-3.68) | 1.16(0.91-1.30) | 1.77(1.68-1.89) |
LC50: Lethal concentration that kills 50% of the exposed adult A. variegatum ticks.
LC90: Lethal concentration that kills 90% of the exposed adult A. variegatum ticks.
Abbreviations: LCL: Lower Confidence Limit; UCL: Upper Confidence Limit.
Table 2: Probit analysis for lethal concentration of three selected botanicals to A. variegatum.
An overall test of efficacy between the treatments has shown that mortality was directly related to the concentration and exposure time. As the concentration of the plant extracts and exposure time increased, the mortality of the tick also increased. This result concurs with whose results showed lower tick mortality with the lower concentrations of 6.25 mg/ml at 1 hr as compared to the higher concentrations. In this current study, the lower concentration (25 and 50 mg/ml) caused lower mortalities while higher concentrations (100 and 150 mg/ml) recorded higher mortalities. Similarly, short exposure time had lower mortality than longer time.
The practical implications of the results presented in this study indicated that efficacy of the extracts increases with increasing concentration and exposure time. Therefore, the present study concluded that P.dodecandra, C. aurea and R. communis could be identified as potential alternative to substitute commercially available chemicals to control A. variegatum.
The authors wish to acknowledge the financial support from Debre Markos University. Similar thanks also go to Machakil Woreda office of veterinary medicine who allowed us to provide valuable information for the study. Lastly, we thank farmers in the locality for allowing sharing their experience for the study.
Citation: Aniley S, Atenafu G (2020) Laboratory Acaricidal Activity of Phytolacca dodecandra, Calpurnia aurea and Ricinus communis against Amblyomma variegatum (Fabricius) (Acari: Ixodidae). Entomol Ornithol Herpetol. 9:237.
Received: 16-Nov-2020 Accepted: 30-Nov-2020 Published: 07-Dec-2020 , DOI: 10.35248/2161-0983.20.9.237
Copyright: © Aniley S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.