Endocrinology & Metabolic Syndrome
Open Access
ISSN: 2161-1017
ISSN: 2161-1017
Case Report - (2022)Volume 11, Issue 3
Immune checkpoint inhibitors (ICIs) significantly increase survival in some malignant tumors, but they also lead to a variety of immune-related adverse events that affect multiple organs, including the liver, lung and endocrine system. Isolated adrenocorticotropic hormone deficiency (IAD), a rare pituitary disorder, may manifest with non-specific symptoms. Clinicians may not be aware of this unusual side effect. We hereby describe a 70-year-old male patient who had lung sarcoma and was treated with camrelizumab. He developed immune-related pneumonia and hepatitis, subsequent late-onset IAD with severe hyponatremia. His symptoms alleviated after glucocorticoid therapy. So regularly monitoring endocrine functions should be beneficial for cancer patients treated by camrelizumab. Clinicians should consider IAD induced by ICIs when hyponatremia occurs in these patients. Timely hydrocortisone replacement therapy can avoid serious, life-threatening adrenal crisis.
Isolated adrenocorticotropic hormone deficiency; Camrelizumab; Lung sarcoma; Hyponatremia
ACTH: Adrenocorticotropic Hormone; TSH: Thyroid-stimulating Hormone; TPOAb: Antithyroperoxidase Antibody; TgAb: Anti-thyroglobulin Antibody; FSH: Follicle Stimulating Hormone; LH: Luteinizing Hormone; GH: Growth Hormone; IGF-1: Insulin-like Growth Factor 1
As a new type of antitumor medications, immune checkpoint inhibitors (ICIs) significantly improve the prognosis of some malignant tumors. However, with their wide applications, many immune-related adverse events (irAEs) related to the ICIs are being realized. irAEs can occur in multiple tissues or organs, including lung, liver and gastrointestinal tract [1,2]. ICIs also cause endocrine dysfunctions [3]. The common endocrine related adverse events are thyroid dysfunctions and hypophysitis, while isolated adrenocorticotropic hormone (ACTH) deficiency (IAD) is rare [3]. IAD has occult symptoms, easy to be misdiagnosed and neglected. Carelizumab (trade name: Erica) is a new type ICI in China [4]. It was officially approved to market in May 2019. The drug has been used to treat many types of malignant tumors. We hereby report a patient with lung cancer who developed severe hyponatremia due to IAD associated with camrelizumab. Meanwhile, we review the characteristics of IAD in order to provide some references for clinical work.
The patient was a 70-year-old male and found to have a pulmonary nodule during his healthy check-up in June 2019. Through computerized tomography (CT) guided biopsy and histopathology, the patient was diagnosed with lung sarcoma and experienced lung local microwave ablation. He had no special family or psycho-social history. The patient had received camrelizumab monotherapy (200 mg every 2 weeks) since July 2019. Nine months later, he started to frequently complain of fever, obvious fatigue, anorexia, nausea and vomiting. He was admitted to our hospital in April 2020. Blood biochemical examination showed hyponatremia (Na 131 mmol/L (137-147 mmol/L)), liver function damage (ALT 139.9 U/L (9-50 U/L), AST 135.1 U/L (15-40 U/L), GGT780.6 U/L (10-60 U/L), TBIL 73.2 μmol/L (5-24 μmol/L)], increased leucocytes and neutrophils (WBC 26.14 × 109/L (3.5-9.5 × 109/L), N 20.13 × 109/L (1.8-6.3 × 109/L)). At that time, plasma ACTH, cortisol level and thyroid function were normal. Chest CT showed multifocal flocculent and cord-like high-density shadows in both lungs and bilateral pleural effusion (Figure 1).
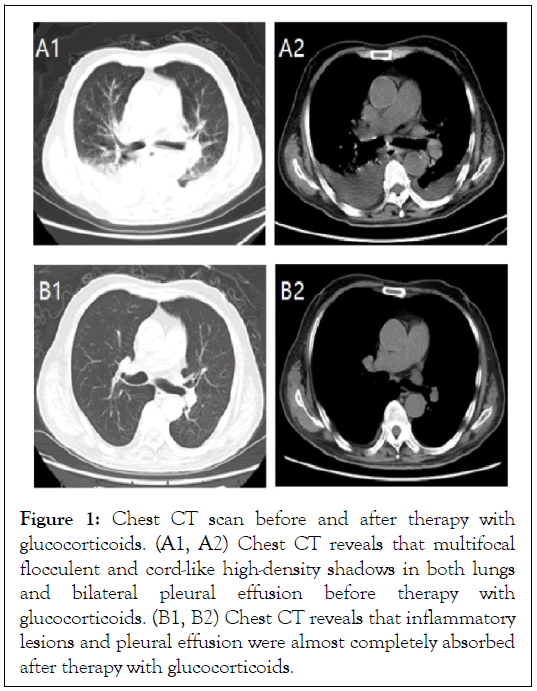
Figure 1: Chest CT scan before and after therapy with glucocorticoids. (A1, A2) Chest CT reveals that multifocal flocculent and cord-like high-density shadows in both lungs and bilateral pleural effusion before therapy with glucocorticoids. (B1, B2) Chest CT reveals that inflammatory lesions and pleural effusion were almost completely absorbed after therapy with glucocorticoids.
We diagnosed the patient with immune-related pneumonia and hepatitis associated with camrelizumab and then discontinued the drug. Methylprednisolone (120 mg QD) was administered to the patient intravenously immediately. Ten days post-infusion, his general condition was rapidly improved. The lesions in the lungs and the pleural effusion almost went away (Figure 1). One month of glucocorticoids therapy later, the hepatic enzyme levels returned to normal range. The patient was discharged from the hospital and gradually terminated glucocorticoids.
Since November 2020, the patient often developed slight fever, anorexia, nausea and vomiting. He was admitted to our hospital again because these symptoms worsened in February 2021. He had been losing weight (over 10 pounds in two months). Laboratory examinations revealed severe hyponatremia (Na 116 mmol/L), but his plasma glucose, liver and renal functions remained normal. Blood routine showed red blood cell 3.54 ×1012 /L [(4.3-5.8) × 1012/L], and hemoglobin 104 g/L [(130-175)g/L]. The hormonal assessment showed that plasma ACTH and cortisol levels at each time point were markedly lower than the normal limits (Table 1).
| Cortisol circadian rhythm | 8 AM | 4 PM | 12 PM |
|---|---|---|---|
| Cortisol (normal range: 172-497) (nmol/L) | 32.65 | 30.85 | 28.16 |
| ACTH (normal range: 5-60) (pg/ml) | 9.55 | 9.78 | 7.44 |
| TSH (normal range: 0.27-4.2) (μIU/mL) | 10.05 | - | - |
| Free T3 (normal range: 3.1-6.8) (pmol/L) | 6.26 | - | - |
| Free T4 (normal range: 12-22) (pmol/L) | 17.63 | - | - |
| TPOAb (normal range: 0-34) (IU/mL) | 8.98 | - | - |
| TgAb (normal range: 0-115) (IU/mL) | 990.3 | - | - |
| FSH (normal range: 1.5-12.4) (IU/L) | 10.83 | - | - |
| LH (normal range: 1.7-8.6) (IU/L) | 8.26 | - | - |
| Prolactin (normal range: 4.04-15.2) (ng/ml) | 29.72 | - | - |
| Testosterone (normal range: 2.8-8.0) (ng/ml) | 5.52 | - | - |
| GH (normal range: 0.06-5.0) (ng/ml) | 1.05 | - | - |
| IGF-1 (normal range: 60-350) (μg/ml) | 82.1 | - | - |
| Plasma renin (normal range: 4.4-46.1) (uIU/ml) | 8.6 | - | - |
| Aldosterone (normal range: 3.0-35.3) (ng/L) | 17.46 | - | - |
Table 1: Hormone assessment showing decreased ACTH and cortisol, elevated TSH and TgAb, elevated prolactin.
Plasma renin activity (PRA) and aldosterone concentration (PAC) were normal. The levels of other anterior pituitary hormones were almost within normal limits (Table 1). Magnetic resonance imaging (MRI) of the pituitary gland showed no abnormalities. Ultrasonography of the thyroid gland and CT of the adrenal glands all revealed normal. Based on these results, he was diagnosed with IAD accompanied by hyponatremia. His symptoms and hyponatremia were dramatically improved after replacement with hydrocortisone 20 mg QD. The patient was regularly followed up after discharge. He has been well up to now, with normal serum sodium levels. Written informed consent was obtained from the patient for publication.
Camrelizumab, as a new type ICI, is increasingly used in clinical anti-tumor therapy. With its wide application, people gradually realize its irAEs. Here we report a rare case that camrelizumab induced immune-related pneumonia and hepatitis, subsequent IAD. It is the first report about IAD caused by camrelizumab. During the treatment of ICIs, the most prevalent endocrine irAEs is thyroid dysfunctions, followed by hypophysitis [3,5]. Thyroid dysfunctions are mainly characterized by asymptomatic thyroiditis. The patient in the case presented subclinical hypothyroidism with the abnormal high levels of TSH and TgAb. It seemed reasonable to diagnose him with immunerelated thyroiditis caused by ICI. Hypophysitis often occurs in men over 60 years old [6]. The clinical symptoms of hypophysitis are usually atypical, such as headache and fatigue [7]. It is often accompanied by multiple hormone deficiency, including TSH, gonadotropin and ACTH [3,8]. But IAD induced by ICIs is very rare, characterized by secondary adrenal insufficiency with low or absent cortisol production but the normal secretion of pituitary hormones other than ACTH. Unlike autoimmune hypophysitis, where a mild to moderate diffuse pituitary enlargement seen in all patients and these morphological alterations can even precede the clinical diagnosis in half of the cases, the pituitary morphology on MRI in IAD induced by ICIs is usually normal. It is possible that the involvement of a single anterior pituitary cell type, in this case corticotroph, is insufficient to develop anatomical alterations that can be seen in imaging tests [9,10]. In addition, although adrenal insufficiency is not associated with mineralocorticoid deficiency, hyponatremia often occurs in IAD as a result of reduced glomerular filtration rate, increased antidiuretic hormone secret and concomitant hypothyroidism [11]. Patients with IAD usually present with non-specific manifestation. Delaying diagnoses and treatment can lead to severe, life-threatening adrenal crisis. Therefore, clinical awareness is essential. Once cancer patients treated with ICIs develop obvious asthenia along with hyponatremia, the diagnosis of IAD should be considered [9,12]. In this case, 1.5 years after the patient was treated with camrelizumab, he developed severe hyponatremia. His hormonal profiles demonstrated hypocortisolemia, low ACTH levels,normal function of other pituitary axes and normal plasma aldosterone levels. These results support the diagnosis of IAD.
Previous studies have described IAD associated with atezolizumab, pembrolizumab and nivolumab therapy, most of which occurred within a few months of initiating ICIs therapy [13-15]. In this case, it was seven months after discontinuation of camrelizumab that the patient developed IAD. Therefore, cancer patients treated with ICIs require regular follow-up tests of serum sodium and endocrine functions not only during the treatment but also after drug withdrawal in order to discover IAD in time. Once IAD occurs, glucocorticoid therapy is essential. The patient’s symptoms soon alleviated after hydrocortisone replacement therapy.
Regularly monitoring serum sodium and endocrine functions is important for cancer patients treated with the ICIs. Early diagnosis of IAD and prompt glucocorticoid therapy can avoid life-threatening adrenal crisis and other serious reactions also. Clinicians should consider IAD induced by ICIs when hyponatremia occurs in this patient. Timely hydrocortisone replacement therapy can avoid serious, life-threatening adrenal crisis.
Ethical approval and consent to participate
“Not applicable”
Consent for publication
Written informed consent has been obtained from the patient for publication of this case report and any accompanying images. A copy can be made available upon request.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Conflict of interests
None to report.
Funding
Not applicable.
Authors’ contributions
Wen Huang is the attending physician of this patient. Wen Huang and Saran Feng are involved in composition and revision of the manuscript.
Not applicable.
Citation: Huang W, Feng S (2022) Isolated Adrenocorticotropic Hormone Deficiency Caused by Camrelizumab in a Patient with Lung Sarcoma: A Case Report and Literature Review. Endocrinol Metab Syndr. 11:360.
Received: 12-Sep-2022, Manuscript No. EMS-22-19715; Editor assigned: 14-Sep-2022, Pre QC No. EMS-22-19715 (PQ); Reviewed: 05-Oct-2022, QC No. EMS-22-19715; Revised: 10-Oct-2022, Manuscript No. EMS-22-19715 (R); Published: 17-Oct-2022 , DOI: 10.35248/2161-0495-22.12.360
Copyright: © 2022 Huang W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.