Orthopedic & Muscular System: Current Research
Open Access
ISSN: 2161-0533
ISSN: 2161-0533
Review Article - (2013) Volume 2, Issue 1
Although it was first described more than 50 years ago, the physiological significance and the potential pathological features of the mu rhythm still remains unclear. In an attempt to clarify certain aspects of this activity, we studied the presence of mu rhythm in 100 healthy subjects with no family history of neurological disease. A 15 minute long resting EEG register was obtained from each subject using a 32 channel Nihon Kodem electroencephalography device, and from these recordings we localized and quantified the mu and alpha rhythms. Kulman´s criteria were used to differentiate the baseline alpha rhythm from mu rhythm within occipital alpha rhythm. Further graphoelements of interest were observed, what leds us to studied the relationship between the mu rhythm and abnormal graphoelements in temporal regions. Our results indicated abnormal graphoelements in 48% of the healthy participants studied here. Neither the mu nor the rolandic alpha rhythms displayed any significant differences between male and females during the appearance of the abnormal graphoelements. However, there was a strong correlation between the appearance of a bilateral mu rhythm and abnormal graphoelements. Despite this temporal association between abnormal graphoelements and rolandic mu rhythm, there is no clear evidence to consider the latter as a pathological sign (no epileptic clinical history within the sample). Nevertheless, the mu rhythm is not necessarily a normal element and further studies will be necessary to clearly define the physiological significance of mu rhythms.
Keywords: EEG; Epilepsy; Graphoelement; Rolandic alpha rhythm; Mu rhythm
“Normality” is purely a statistical concept and as such, it is extremely difficult to establish what is and what is not “normal” in electroencephalography (EEG) recordings. When electroencephalography recording was first introduced [1,2] four basic rhythms were characterized through their specific temporal and topographical appearance: alpha, beta, delta and theta. Since then, each and every variation with respect to these four basic rhythms is conceived as “not normal” [3]. Years later, a new characteristic rhythm was described as a “an arch shaped rhythm, with a spiky shape (rhythm en arceau)”, the mu rhythm (Figure 1). Although this mu rhythm was originally considered to be a non-pathological event, it is nowadays assumed to be somehow connected with a certain level of neuronal hyperexcitability [4]. Therefore, mu rhythms may coexist with psychopathological symptoms, such as anxiety, aggressiveness, hyperactivity and other psychosomatic features [5]. Clinical experience led us to predict different types mu rhythm associated to psychopathological symptoms.
Following the description of mu rhythms in adults, they were subsequently identified in children [6] and animals [7], such as cats and monkeys. The degree and quality of attention on the one hand, and immobility on the other, seem to be the two main factors that provoke or maintain mu rhythms [6]. However, in all circumstances this mu rhythm is more visible in rolandic-parietal than in rolandicfrontal regions. Moreover, a “third” mu rhythm was recently described in humans that serves to attenuate the time that the eyes are opened [8] and subsequently, different types of beta rhythms below 30 Hz have been shown to exist [9]. More recently, it was also proposed that the mu rhythm is associated with comprehension and learning process in humans, and that it may have further implications in cognitive performance [10].
In terms of its pathological implications, the presence of mu rhythm was analyzed in a sample of more than 500 volunteers, 53 displaying clinical symptoms of epilepsy. In this cohort, it appeared that the mu rhythm is more frequently located in the hemisphere of the epileptic focus, and it displays more severe characteristics in such participants [11]. Furthermore, in this study the prognosis for temporal foci were better than for those with front-central foci. Conversely, the mu rhythm was not identified in 33 healthy controls, which suggested its association with epileptic crises, even in the absence of other clinical complications (Figure 2).
In an attempt to clarify the true significance of the rolandic mu rhythm in healthy population, we have carried out a large electroencephalographic study in which we unexpectedly found abnormal temporal epileptiform graphoelements. These features were to some extent related with arch shaped rolandic waves, which were also studied.
Participants
A cohort of 100 young adults was studied, aged from 17 to 26 years old ( 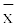 = 19.39 ± 2.07: 48 male,
= 19.39 ± 2.07: 48 male, 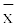 = 19.9 ± 2.46; 52 female,
= 19.9 ± 2.46; 52 female, 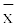 =18.7 ± 1.26), made up of University of Murcia (Spain) Medical School students with no personal or family history of neurological disorders. All of them signed an informed consent form before participating in the study, which was approved by the ethic committee of the Nuestra Sra. del Rosell University Hospital (Cartagena, Murcia, Spain) and that conformed with the Helsinki protocol of 1975. The participation in the study involved completing a demographical data questionnaire. Furthermore, all of them underwent a standard 15 minutes eyes closed EEG recording study within the hospital facilities. Participants were asked to relax and follow some orders as alternating “hand fisted” and “hand open”.
=18.7 ± 1.26), made up of University of Murcia (Spain) Medical School students with no personal or family history of neurological disorders. All of them signed an informed consent form before participating in the study, which was approved by the ethic committee of the Nuestra Sra. del Rosell University Hospital (Cartagena, Murcia, Spain) and that conformed with the Helsinki protocol of 1975. The participation in the study involved completing a demographical data questionnaire. Furthermore, all of them underwent a standard 15 minutes eyes closed EEG recording study within the hospital facilities. Participants were asked to relax and follow some orders as alternating “hand fisted” and “hand open”.
Apparatus
EEG studies were carried out using a Nihom Kodem EEG-1100 Neurofax apparatus with 12 out of 32 electrodes available configured in bipolar longitudinal and transversal patterns according to the 10-20 schemes. The electrodes were placed a suitable distance apart from each other in order to avoid signal interaction and the parameters selected were: time constant, 0.3 sec; frequency, 260 Hz; amplification, 100µV/ cm. The maximum EEG recording time was 15 minutes, recorded under relaxed awake conditions.
Analysis
The EEG was first analysed visually by trained neurophysiologist to discharge clinical participants. The intrasubject variables analysed were age and sex. Fast Fourier Transformation (FFT) analysis was conducted in 5 secs periods to identity alpha, alpha rolandic, mu rhythm and abnormal graphoelements frequencies and locations. We defined abnormal graphoelements as EEG activity that interrupts the stationality in the brain electric trace that changes the dominant frequencies for less than 2 seconds, EEG amplitude doubles the baseline rhythm in any of its components, usually in a manner involving the formation of peaks (Figure 3). Furthermore, rhythm frequency and morphology within the rolandic area during the abnormal graphoelement appearance were identified. Only the more frequent abnormal graphoelements that lasted longer than 1 second were analysed, i.e., those observed more than 5 times during the 15 minute trace. Alpha and mu rhythms were identified according to Kuhlman [12,13]. Independent ANOVA analysis was conducted to evaluate the significance of different factors on the appearance of abnormal graphoelements, mu and alpha rhythms.
Among the participants assessed, alpha rhythm was identified within the parietal-occipital areas of 97% of the participants (Table 1), with an average frequency of 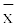 =10.28 ± 0.8 Hz (Figure 4). In 94% of these participants, these alpha rhythms were located occipitally, with an average frequency of 10.16 ± 0.8 Hz in males and 10.39 ± 0.8 Hz in females. There was no significant relationship between gender and alpha frequency within this group (p>0.005).
=10.28 ± 0.8 Hz (Figure 4). In 94% of these participants, these alpha rhythms were located occipitally, with an average frequency of 10.16 ± 0.8 Hz in males and 10.39 ± 0.8 Hz in females. There was no significant relationship between gender and alpha frequency within this group (p>0.005).
| Gender | % | Frequency HZ | P | Sig. |
|---|---|---|---|---|
| Total | 94 | 10.28 | .8 | n.s |
| Male | 95.95 | 10.16 | .78 | n.s |
| Female | 97.95 | 10.39 | .65 | n.s |
| Total | 76 | 11.37 | .86 | n.s |
| Male | 74.96 | 11.37 | .77 | n.s |
| Female | 76.9 | 11.78 | .9 | n.s |
Table 1: Alpha and mu rhythm values within the sample (%).
The rolandic rhythm was identified within 76% of the trace (Table 1) and with arch shaped waves in 66% of these, which responded well to contralateral hand movement. In function of sex, the frequencies were 11.37 ± 0.86 Hz for males and 11.78 ± 0.72 Hz for females, and thus, there was no significant effect of sex on rolandic frequencies (p>.005, Figure 5). The rolandic rhythm amplitude oscillated between 20 and 40 µV, although it reached up to 70 µV. The alpha rolandic rhythm was situated bilaterally (corresponding electrodes, i.e. T3 and T4) in 36.12% of the cohort, exclusively within the right hemisphere in 23.80% and only within the left hemisphere in 16.08% of the sample.
Abnormal graphoelements appeared in 87% of the sample, and they were not affected by sex factor (p>0.005), appearing in 45% of males and 52% of females. Bilateral outbreaks of abnormal graphoelements were evident in 27% of the cohort, 31% in the right temporal lobe and 41.6% in the left temporal lobe. The rolandic frequency in temporal regions during these outbreaks of abnormal graphoelements was 9.8 ± 0.85 Hz, displaying a tendency towards a lower than baseline rolandic frequency that did not reach statistically significance (p>0.001) [14,15]. However, there is a interaction between the appearance of mu rhythm and abnormal graphoelements both unilateral (Figure 6 and Table 2; p<0.001) and bilaterally (p<0.001).
| Mu | Epileptic focus | No epileptic focus |
|---|---|---|
| Yes | 42% | 34% |
| No | 6% | 18% |
| Total | 48% | 52% |
Table 2: Proportion of mu rhythm and abnormal graphoelements simultaneous appearance within the sample.
The results obtained here indicate a higher frequency of abnormal EEG graphoelements in a healthy young adult population than found previously [16-19], a higher frequency that could not be attributed to a genetic origin [20] since one of the exclusion criteria was the existence of a familiar history of neurological and/or psychodiseases. Accordingly, it seems more likely that the explanation for this difference supports Carreño’s theory [21] whereby most of the peak-wave outbursts are located within temporal regions. It is also improbable that the abnormal brain traces were due to impaired CNS development as they were obtained from qualified university students.
Although the abnormal bioelectrical signs were not directly related with epileptic tendencies in the individuals [22], it is likely they are related to a certain tendency towards cortico-electrical instability [23]. The reason why these abnormal bioelectrical features were not associated with epileptic crisis may have been due to the activation of inhibitory barriers, as described previously [24-27].
In terms of the mu rhythm, we believe that this corresponded to the mu rhythm described by Covello (8), which was frequent during vigilance and that did not always present an arch shaped morphology. Since this pattern usually appeared within the alpha rhythm, it was referred to as the “rolandic alpha rhythm” [28]. Our results indicated that the abnormal frequencies were common, like those observed by Kuhlman [12], although this earlier cohort was much smaller than that studied here. We believe there are two main reasons why there was a high frequency of mu rhythms found in our sample: 1) we didn´t look for a specific morphology but rather, a specific reactive alpha rhythm located within the rolandic region (as opposed to the occipital alpha region); 2) Our electrode pattern was based on longer inter-electrode spaces than those used in previous studies, reflecting the relationship between “inter-electrode distance and trace amplitude”. Nevertheless, our results confirm previous data regarding interhemispheric trace variation [29] and regarding the dominant frequency [12], particularly given the higher frequency of the rolandic alpha regions with respect to the occipital alpha regions.
Regarding the location of the rhythms, in our study we found the mu rhythm in the pre-rolandic area, while the vast majority of earlier studies found it in post-rolandic areas [30], which is why it was called “somatosensitive alpha”. Those differences in location may be due to our different criteria when obtaining the EEG registers.
In reference to the arch-shaped wave morphology, this appeared in 60% of the cohort with clear appearance of rolandic alpha rhythm that reached amplitude of 80 µV, although not all of these participants presented temporal brushes of abnormal graphoelements temporal. Furthermore, the absence of a rolandic alpha rhythm was not synonymous with the absence of abnormal graphoelements. The alpha rhythm may be strongly influenced by attention phenomena and under such conditions; the alpha rhythm disappears from the trace. However, it has been stated that it is possible to find rolandic alpha rhythms in all participants through frequency analysis.
Some authors have previously related mu rhythm to cognitive processes; mu rhythm is not a pathological sign per se. It rather seems in relation to the integration of information at somatosensory, somatosensitive and motor brain areas. It is possible that the appearance of the mu rhythm and the occurrence of abnormal graphoelements in temporal regions may be related to anxiety or emotional sensitivity in the cohort studied [6]. However, its coincidence with the appearance of abnormal graphoelements and the electrical slowness represents a clear sign of cortical irritability or instability [15], which could occur in the absence of epileptic phenomenon. We would hypothesise that this process may be in connexion to comprehension and learning processes instead of pathological ones. In our experience, pathological mu rhythm use to be more persistent and associated to further pathological symptoms. Further studies on larger cohorts would probably identify different types of mu rhythm with variable pathological significance [31,32]. Therefore, in the light of our current data it seems risky to accept the aforementioned theory of Saradzhishvili [33] and in our opinion, the arch shaped wave morphology is most likely related to the morphology of the source area and to its predominant rhythm at that time [12]. This arch shaped wave was not associated to abnormal graphoelements. However, that is the case in our sample, even bilaterally. In our opinion. Those signs are not epileptogenic. In summary, we propose that the high frequency co-appearance of roandic arch shaped wave and abnormal graphoelements may be in strong relation to learning, attention (our participants were highly qualified students), as supported by previous studies (Ganis and Cutas, 2003 ). Tiredness may be an alternative origin for EEG abnormal graphoelements [34,35]. It has been described the implication of posterior prefrontal basal cortex as a mediator in tiredness management [36-39], probably engaged with emotional factors (Brodal et al., 1992). Further studies are required to a better description of this phenomenon.
Rolandic alpha rhythms appeared in 76% of a healthy population of individuals, 48% of whom displayed abnormal temporal graphoelements. In 87% of this population, there was a significant association between the presence of abnormal graphoelements and mu morphology in rolandic alpha rhythms. The absence of mu rhythms was associated with the absence of abnormal graphoelements, although there was insufficient evidence to sustain that abnormal temporal graphoelements were necessarily synonymous with an electrical pathology in the brain, even when associated to rolandic mu rhythms. It is possible that the studies with electrical brain maps, with basal interpolation possibilities can aid in the clearance of de problem with non invasive and non pensive techniques.