
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2020)Volume 11, Issue 3
Background: Patients with primary immunodeficiency disorders (PIDD) typically require life-long immunoglobulin
(IG) replacement therapy. There are two routes of IG administration: intravenous (IVIG) and subcutaneous (SCIG).
To better understand routes of IG administration in the real-world, use in a home infusion setting was evaluated.
Objective: To evaluate the safety, efficacy, and perceived responses in PIDD patients on IVIG versus SCIG in a realworld,
home infusion setting.
Methods: Retrospective data were collected from 2010 to 2018 from a national home infusion pharmacy of PIDD
patients receiving IVIG or SCIG therapy for at least 6 months with evaluations for safety, efficacy, and patient
perception of response.
Results: A total of 149 patients were identified for analysis: IVIG (n=84) and SCIG (n=65). Overall, patients in the
SCIG group had higher rates of local adverse reactions, while patients receiving IVIG had higher rates of systemic
adverse reactions. Both SCIG and IVIG were effective as the majority of patients had ≤ 1 infection or hospital visit
within the study period. However, patients in the SCIG group had fewer hospital visits and lower rates of infections
overall. Patients receiving SCIG also perceived a faster speed of response.
Conclusion: SCIG infusions are safe, efficacious, and well tolerated when compared to IVIG, providing PIDD
patients with an alternative route of IG administration. Notably, hospital visits and infection rates were significantly
reduced in patients receiving SCIG. The overall findings of this study contribute to growing evidence that
demonstrates the benefits of SCIG in adult and pediatric patients with PIDD.
IVIG; SCIG; Immunoglobulin; Safety; Primary immunodeficiency; PIDD; Home infusion; Efficacy; Patient-reported outcomes
Primary immunodeficiency disorders (PIDD) include a heterogenous group of inherited disorders with deficiencies in one or more components of the immune system, which increases a patient ’ s susceptibility to infections [1-3]. There are approximately 350 distinct forms of PIDD [1,4,5]. Life expectancy is reduced and recurrent infections cause significant morbidity and disability due to various complications including chronic lung disease, inflammatory bowel disease, and autoimmune disorders [2,6,7]. Therefore, patients with PIDD require life-long immunoglobulin (IG) replacement therapy to prevent recurrent infections, notably bacterial infections of the respiratory tract [3,6-9].
Currently, there are two routes of IG administration in the United States (US): intravenous (IVIG) and subcutaneous (SCIG). In the early 1980s, IVIG was introduced in the US and adopted as the standard treatment at the time [10,11]. SCIG was initially introduced in 1952 by Colonel Bruton who used a 16% solution for the treatment of a boy with agammaglobulinemia which demonstrated a beneficial effect [12]. Because SCIG infusions were relatively slow, and the volume that could be infused in a single infusion was limited, the IVIG route became more popular in the US. However, during the 1990s, SCIG slowly gained popularity among patients, with the first SCIG product approval in 2006 [13,14]. Since the advent of IG therapies, there has been a decrease in morbidity from infections, increased survival, and improvement in overall quality of life (QoL) [2,9]. Survival of patients with antibody deficiencies has increased dramatically since the introduction of IG replacement therapy, and the efficacy of IVIG and SCIG in preventing serious bacterial infections is well established [2,6,7,13,14].
Clinical trials of SCIG administered weekly have shown comparable efficacy versus IVIG in preventing and/or minimizing serious bacterial infections, hospitalizations due to infection, days missed from work/school, and days on antibiotics in patients with antibody deficiency [2,9,14]. Although clinical studies have demonstrated comparable efficacy and tolerability between IVIG and SCIG, there is limited literature published using real-world data [13,15]. To better understand the use of these therapies in a real-world, home infusion setting, our study objectives were to evaluate the comparative safety, efficacy, and outcome perceptions in PIDD patients treated with IVIG and/or SCIG in the US.
Study design and data source
A retrospective observational study was conducted using patient electronic medical records (EMR) provided by KabaFusion, LLC, (KabaFusion) a national home infusion provider that specializes in IG therapy. KabaFusion has pharmacies throughout the country and provides patient-focused services in 40 states. Study data included therapy information (e.g., type of therapy, route of administration, dose, number of infusions), switching rates, patient characteristics (e.g., age, diagnosis, gender, state of residence, comorbidities), local/systemic adverse reactions, hospital visits (both outpatient and inpatient visits), types of infections, and patient perception of response. The protocol for this study was assessed by an Independent Review Board (IntegReview) and received an exemption according to 45 CFR 46.101(b).
Study population
Data from adult and pediatric patients with PIDD, who received IVIG or SCIG therapy from KabaFusion, LLC for at least 6 months (between July 1, 2010 and July 31, 2018) were selected for inclusion in this study. Patients with incomplete data (within the medical record assessments) or missing data (due to patients being discharged or transferred to another home infusion provider) were excluded from this study.
Study outcomes
There were three main study outcomes assessed between the two study groups: safety, efficacy, and patient perception of response. Safety outcomes were defined as percentages of patients receiving IVIG versus SCIG who experienced local and systemic adverse reactions. Efficacy outcomes were defined as rates of hospital visits, incidence of infection, and types of infections. Perception of response outcomes were gathered and recorded in a patient’s EMR by the infusion nurse or pharmacist. Due to the retrospective nature of the study, these outcomes were not available for every patient. However, recorded variables included energy levels (low, normal/moderate, or high), pain (scale of 0-10), gastrointestinal symptoms (yes/no), and perception of speed of response. The speed of response was noted as the time period between the start of therapy and the patient’s perception of significant improvement in symptoms (e.g., general health/ well-being, fewer recurrent infections, and/or fewer antibiotic prescriptions). These changes were reported to either a nurse or a pharmacist and recorded in the patient’s EMR.
Statistical analysis
Baseline demographics were recorded prior to the start of IVIG or SCIG therapy. Unadjusted descriptive statistics were conducted to summarize the demographics between the two study groups, such as mean and standard deviation (SD) for continuous variables, and percentages for categorical variables. Statistical differences were evaluated using Student’s t test for continuous variables; chi-square and Fisher’s exact tests were used for categorical variables. Analyses were conducted using SAS 9.4 software (SAS Institute, Cary, North Carolina). An unpaired two-sided p value <0.05 was considered statistically significant.
Study population and baseline characteristics
There were 149 patients identified for inclusion in this study: 84 patients (56.4%) were on IVIG and 65 patients (43.6%) were on SCIG (Table 1). Overall, there were more females (n=105 (70.5%)) than males (n=44 (29.5%)). The overall mean age was 49.7 years (SD: 21.1). In the IVIG group, the mean age was 50.0 years (range: 8-84 years (SD: 19.6)), while in the SCIG group it was 49.4 years (range: 4-86 years (SD: 23.0)). The majority of patients (n=60 (40.3%)) resided in the Northeast region of the US (p=0.002).
| Patient Characteristics | Patients on IVIG | Patients on SCIG | p value |
|---|---|---|---|
| n=84 | n=65 | ||
| Gender, n (%) | 0.421 | ||
| Male | 23 (27.4%) | 21 (32.3%) | |
| Female | 61 (72.6%) | 44 (67.7%) | |
| Age, years | |||
| mean (SD) | 50.0 (19.6) | 49.4 (23.0) | 0.526 |
| (min-max) | (8-84) | (4-86) | |
| Geographic Region, n (%) | 0.002 | ||
| Midwest | 3 (3.6%) | 5 (7.7%) | |
| Northeast | 30 (35.7%) | 30 (46.2%) | |
| South | 19 (22.6%) | 16 (24.6%) | |
| West | 32 (38.1%) | 14 (21.5%) | |
| Immunodeficiency Diagnosis, n (%) | |||
| CVID | 60 (71.4%) | 47 (72.3%) | 0.612 |
| Selective IG Deficiency | 8 (9.5%) | 7 (10.8%) | 0.521 |
| Immunity Deficiency NOS | 2 (2.4%) | 1 (1.5%) | 0.529 |
| Combined Immunodeficiency | 1 (1.2%) | 1 (1.5%) | 0.537 |
| Nonfamilial Hypogammaglobulinemia | 13 (15.5%) | 8 (12.3%) | 0.049 |
| Hyperimmunoglobulin E syndrome | 0 (0.0%) | 1 (1.5%) | - |
| Comorbidities, n (%) | |||
| Respiratory Issues* | 48 (57.1%) | 33 (50.8%) | 0.445 |
| Cardiac Issues | 10 (11.9%) | 6 (9.2%) | 0.018 |
| Congestive Heart Failure | 2 (2.4%) | 1 (1.5%) | 0.291 |
| Diabetes | 11 (13.1%) | 9 (13.8%) | 0.932 |
| Dyslipidemia | 11 (13.1%) | 12 (18.5%) | 0.029 |
| Hypertension | 24 (28.6%) | 19 (29.2%) | 0.901 |
| Stroke | 1 (1.2%) | 0 (0.0%) | - |
Abbrevations: IVIG: Intravenous immunoglobulin; SCIG: Subcutaneous immunoglobulin; N/n: Number; SD: Standard deviation; CVID: Common variable immune deficiency; IG: Immunoglobulin; NOS: Not otherwise specified.
p value <0.05 considered statistically significant
*: Includes asthma, chronic obstructive pulmonary disorder, and otherwise unspecified respiratory disorders
Table 1: Baseline patient characteristics by route of administration.
Among total PIDD diagnosis categories, the majority of patients (n=107 (71.8%)) had a diagnosis of CVID. Noteworthy differences in immunodeficiency diagnosis between IVIG and SCIG groups were observed in the incidence of nonfamilial hypogammaglobulinemia (IVIG n=13 (15.5%) versus SCIG n=8 (12.4%))(p=0.049). A high number of patients with comorbid chronic respiratory disease overall (n=61 (40.9%)) with a similar distribution between the IVIG (n=48 (57.1%)) and SCIG (n=33 (50.8%)) groups (p=0.445). Notable differences between both treatment groups were observed in patients with comorbid cardiac issues (IVIG n=10 (11.9%) versus SCIG n=6 (9.2%)) (p=0.018) and dyslipidemia (IVIG n=11 (13.1%) versus SCIG n=12 (18.5%))(p=0.029).
Therapy and switching information
The majority of patients (n=124 (83.2%)) did not switch routes of administration (Table 2). However, if patients switched, most switched from IVIG to SCIG (n=16 (10.7%)) versus from SCIG to IVIG (n=6 (4.0%)). A very small number of patients switched back and forth between the different routes of administration (n=3 (2.0%)). The mean duration of treatment was similar between the two groups (IVIG=23.80 months versus SCIG=23.68 months) (p=0.920). However, when the months were categorized by time (<12 months, 12 months to <24 months, etc.), the distribution of patients became statistically significant (Table 2). A greater proportion of SCIG patients had longer durations of therapy (≥36 months) versus IVIG patients (p<0.001) (Table 2).
| Therapy Information | Patients on IVIG | Patients on SCIG | p value |
|---|---|---|---|
| n=84 | n=65 | ||
| Switching Rates, n (%) | |||
| Patients switched from IVIG to SCIG | 0 (0.0%) | 16 (24.6%) | - |
| Patients switched from SCIG to IVIG | 6 (7.1%) | 0 (0.0%) | - |
| Patients switched back and forth between routes | 2 (2.4%) | 1 (1.5%) | 0.621 |
| No switching occurred | 76 (90.5%) | 48 (73.8%) | 0.029 |
| Change in Dosage/Month, n (%) | |||
| No change in dosage | 59 (70.2%) | 46 (70.8%) | 0.882 |
| ≥ 1 change in dosage | 25 (29.7%) | 19 (29.2%) | 0.812 |
| Duration of Therapy (Months), n (%) | |||
| mean (SD) | 23.80 (19.76) | 23.68 (19.08) | 0.92 |
| <12 months | 28 (33.3%) | 27 (41.5%) | 0.04 |
| 12 months to <24 months | 26 (31.0%) | 16 (24.6%) | 0.027 |
| 24 months to <36 months | 16 (19.0%) | 7 (11.0%) | <0.001 |
| ≥ 36 months | 13 (15.0%) | 15 (23.0%) | <0.001 |
| Duration missing | 1 (1.2%) | 0 (0.0%) | - |
Abbrevations: IVIG: Intravenous immunoglobulin; SCIG: Subcutaneous immunoglobulin; N/n: Number.
p value <0.05 considered statistically significant
Table 2: Therapy information by route of administration.
Safety outcomes
Patients in the SCIG group had higher rates of local adverse reactions versus the IVIG group, including local infusion site edema (SCIG n=3 (4.6%) versus IVIG n=2 (2.4%))(p=0.022), non-specific patient-reported infusion site reactions (SCIG n=15 (23.1%) versus IVIG n=8 (9.5%))(p<0.001), and infusion site pain (SCIG n=3 (4.6%) versus IVIG n=2 (2.4%)) (p=0.022) (Figures 1 and 2) (Table 3).
| Safety Outcomes | Patients on IVIG | Patients on SCIG | p value |
|---|---|---|---|
| n=84 | n=65 | ||
| Local adverse reactions, n (%) | |||
| Edemas | 2 (2.4%) | 3 (4.6%) | 0.022 |
| Infusion site reaction | 8 (9.5%) | 15 (23.1%) | <0.001 |
| Infusion site pain | 2 (2.4%) | 3 (4.6%) | 0.022 |
| Bruising | 2 (2.4%) | 2 (3.1%) | 0.532 |
| Systemic adverse reactions, n (%) | |||
| Headache | 29 (34.5%) | 10 (15.4%) | <0.001 |
| Fatigue | 19 (22.6%) | 8 (12.3%) | <0.001 |
| Shortness of breath | 7 (8.3%) | 7 (10.8%) | <0.001 |
| Fever | 8 (9.5%) | 5 (7.7%) | 0.027 |
| Flu-like symptoms | 10 (11.9%) | 3 (4.6%) | <0.001 |
| Muscle pain | 9 (10.7%) | 4 (6.2%) | <0.001 |
| Nausea | 8 (9.5%) | 5 (7.7%) | <0.001 |
| Vomiting | 6 (7.1%) | 2 (3.1%) | <0.001 |
| Dizziness | 5 (6.0%) | 3 (4.6%) | <0.001 |
| Urticaria | 2 (2.4%) | 4 (6.2%) | <0.001 |
| Rash | 2 (2.4%) | 4 (6.2%) | <0.001 |
| Increased blood pressure | 3 (3.6%) | 2 (3.1%) | 0.573 |
| Pulmonary congestion | 2 (2.4%) | 3 (4.6%) | 0.022 |
| Respiratory tract infections | 2 (2.4%) | 2 (3.1%) | 0.591 |
| Sinusitis | 2 (2.4%) | 2 (3.1%) | 0.591 |
| Chills | 2 (2.4%) | 1 (1.5%) | 0.424 |
| Diarrhea | 2 (2.4%) | 1 (1.5%) | 0.424 |
| Hives | 1 (1.2%) | 2 (3.1%) | 0.038 |
| Flushing | 1 (1.2%) | 1 (1.5%) | 0.72 |
| Rigors | 2 (2.4%) | 0 (0.0%) | - |
| Rhinitis | 0 (0.0%) | 1 (1.5%) | - |
Abbrevations: IVIG: Intravenous immunoglobulin; SCIG: Subcutaneous immunoglobulin; N/n: Number.
p value <0.05 considered statistically significant
Table 3: Safety outcomes (local and systemic effects) by route of administration.
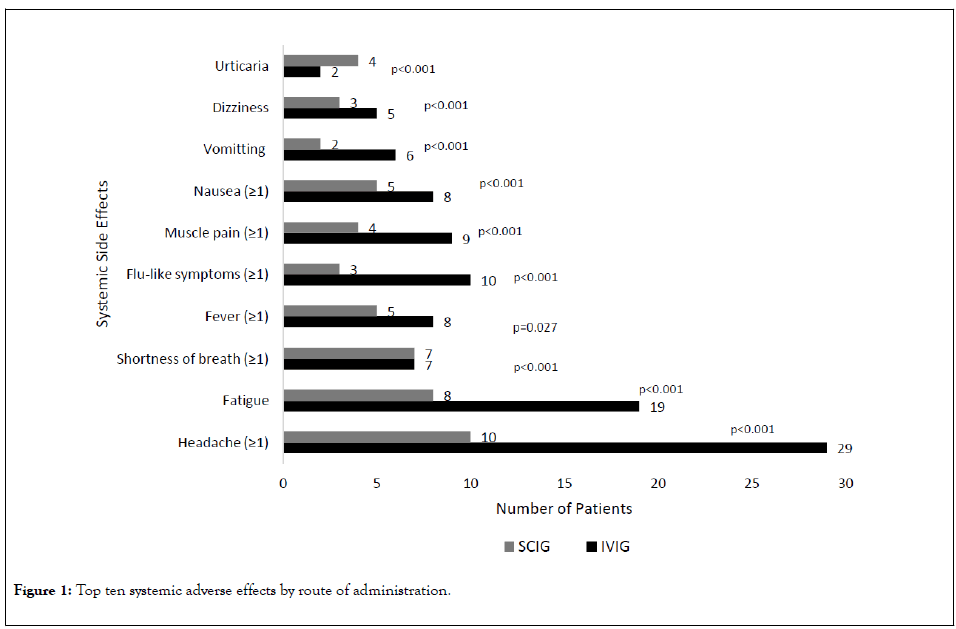
Figure 1: Top ten systemic adverse effects by route of administration.
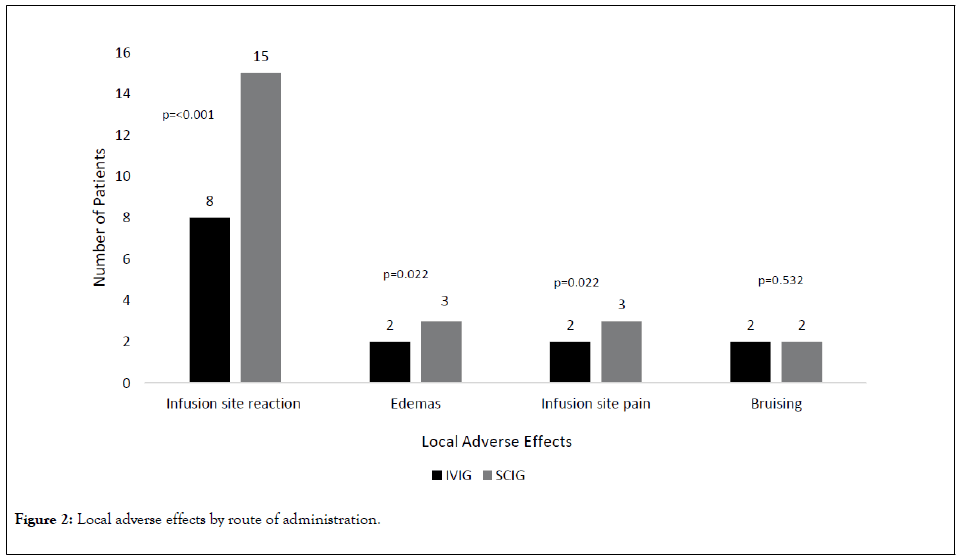
Figure 2: Local adverse effects by route of administration.
The trend was reversed when evaluating systemic adverse reactions. The IVIG group had higher rates of systemic adverse reactions versus the SCIG group, including headache (IVIG n=29 (34.5%) versus SCIG n=10 (15.4%))(p<0.001), fatigue (IVIG n=19 (22.6%) versus SCIG n=8 (12.3%))(p<0.001), and fever (IVIG n=8 (9.5%) versus SCIG n=5 (7.7%))(p=0.027) (Figures 1 and 2)(Table 3).
Efficacy outcomes
Overall, both SCIG and IVIG had high efficacy rates in terms of preventing hospital visits and infections. The majority of patients had 0 hospital visits (n=121 (81.2%)) and ≤ 1 infection (n=110 (73.8%)) (Table 4). However, patients in the SCIG group had significantly lower rates of hospital visits (n=11 (16.9%)) versus patients in the IVIG group (n=17 (20.2%))(p<0.001). SCIG patients had a greater proportion of 1 or 2 hospital visits (Table 4), but IVIG patients had a higher proportion of ≥ 3 hospital visits (n=6 (7.1%)) as compared to 0 in the SCIG group. The SCIG group also had lower rates of ≥ 1 infection versus IVIG patients (n=26 (40.0%) versus n=44 (52.4%), respectively) (p<0.001).
| Efficacy Outcomes | Patients on IVIG | Patients on SCIG | p value |
|---|---|---|---|
| n=84 | n=65 | ||
| Rates of Hospital Visits, n (%) | |||
| 0 visits | 67 (79.8%) | 54 (83.0%) | 0.328 |
| Hospital visits (≥ 1) | 17 (20.2%) | 11 (16.9%) | <0.001 |
| 1 visit | 7 (8.3%) | 7 (10.8%) | 0.017 |
| 2 visits | 4 (4.8%) | 4 (6.2%) | 0.021 |
| ≥ 3 visits | 6 (7.1%) | 0 (0.0%) | - |
| Rates of Infection, n (%) | |||
| 0 infections | 40 (47.6%) | 39 (60.0%) | <0.001 |
| Infection rate (≥ 1) | 44 (52.4%) | 26 (40.0%) | <0.001 |
| 1 infection | 20 (45.5%) | 11 (42.3%) | 0.727 |
| 2 infections | 11 (25.0%) | 9 (34.6%) | <0.001 |
| 3 infections | 2 (4.5%) | 3 (11.5%) | <0.001 |
| 4 infections | 4 (9.1%) | 1 (3.8%) | <0.001 |
| ≥ 5 infections | 7 (8.3%) | 2 (3.1%) | - |
| Types of Infections*, n (%) | 44 (52.4%) | 26 (40.0%) | <0.001 |
| Sinus | 37 (84.1%) | 22 (84.6%) | 0.881 |
| Respiratory | 30 (68.2%) | 22 (84.6%) | <0.001 |
| Viral | 11 (25.0%) | 7 (26.9%) | 0.731 |
| Bladder | 8 (18.2%) | 3 (11.5%) | <0.001 |
| Skin | 7 (15.9%) | 3 (11.5%) | <0.001 |
| Bacterial | 5 (11.4%) | 2 (7.7%) | <0.001 |
| Ear | 4 (9.1%) | 2 (7.7%) | 0.042 |
| Unspecified | 4 (9.1%) | 2 (7.7%) | 0.042 |
| Gastrointestinal | 1 (2.3%) | 4 (15.4%) | <0.001 |
| Eye | 2 (4.5%) | 1 (3.8%) | 0.481 |
| Oral | 3 (6.8%) | 0 (0.0%) | - |
| Bone | 1 (2.3%) | 0 (0.0%) | - |
| Staph | 1 (2.3%) | 0 (0.0%) | - |
| Yeast | 0 (0.0%) | 1 (3.8%) | - |
Abbrevations: IVIG: Intravenous immunoglobulin; SCIG: Subcutaneous immunoglobulin; N/n: Number
*: Not mutually exclusive
p value <0.05 considered statistically significant
Table 4: Efficacy outcomes by route of administration.
In the overall population, the most frequent infections included sinus (n=59 (84.3%)), respiratory (n=52 (74.3%)), viral (n=18 (25.7%)), renal/urinary (n=11 (15.7%)), and skin/subcutaneous (n=10 (14.3%)) (Figure 3). Sinus and viral infections were not significantly different between the two groups, but patients receiving IVIG had lower rates of respiratory infections than SCIG patients (n=39 (68.2%) versus n=22 (84.6%), respectively) (p<0.001), while patients receiving SCIG had lower rates of skin/subcutaneous and renal/urinary infections than IVIG patients (p<0.001).
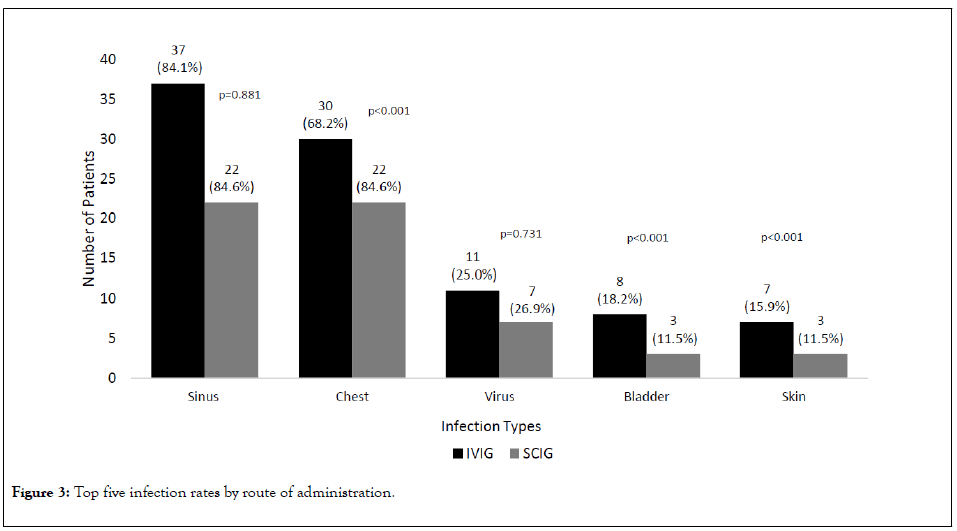
Figure 3: Top five infection rates by route of administration.
Patient outcomes and perception of response
Overall, 27 (18.1%) patients reported their energy levels (Table 5) which were rated as low, normal/moderate, or high. Although the number of reported outcomes is small, 11.1% of patients in the IVIG group reported low energy levels versus no patients reporting low energy levels in the SCIG group. Approximately 77.8% of SCIG patients reported normal/ moderate energy levels versus 66.7% of patients in the IVIG group (p=0.027). Data for pain were available for 33 (22.1 %) patients as rated on a scale of 0 (no pain) to 10 (worst possible pain). Notably, there was no pain levels reported >5, and all of the patients reporting a 5 (on the pain scale) occurred in the IVIG group (29.4%). Approximately 87.5% of patients in the SCIG group had a pain scale level of 0 compared to 58.8% of patients in the IVIG group (p=0.002). Similarly, fewer patients in the SCIG group experienced pain levels of 1 and 4 compared to IVIG patients. The majority of patients didn’t experience any gastrointestinal symptoms (84.6%). However, there were 16 (19.0%) patients in the IVIG group and 7 (10.8%) patients in the SCIG group that experienced gastrointestinal symptoms (p=0.021). The mean speed of patient perception of response was faster for patients in the SCIG group (4.0±3.5 months) versus those in the IVIG group (4.9±4.9 months)(p<0.001).
| Patient Outcomes | Total Patients | Patients on IVIG | Patients on SCIG | p value |
|---|---|---|---|---|
| N=149 | n=84 | n=65 | ||
| Current Energy Level, n (%) | ||||
| Energy level, not reported | 122 (81.9%) | 66 (78.6%) | 56 (86.2%) | <0.001 |
| Energy level, reported | 27 (18.1%) | 18 (21.4%) | 9 (13.8%) | |
| Low | 2 (7.4%) | 2 (11.1%) | 0 (0.0%) | - |
| Normal/Moderate | 19 (70.4%) | 12 (66.7%) | 7 (77.8%) | 0.027 |
| High | 6 (22.2%) | 4 (22.2%) | 2 (22.2%) | 0.981 |
| Pain Scale (0-10), n (%) | ||||
| Pain, not reported | 116 (77.9%) | 67 (79.8%) | 49 (75.4%) | 0.628 |
| Pain, reported | 33 (22.1%) | 17 (20.2%) | 16 (24.6%) | |
| Level 0 | 24 (72.7%) | 10 (58.8%) | 14 (87.5%) | 0.002 |
| Level 1 | 3 (9.1%) | 2 (11.8%) | 1 (6.25%) | <0.001 |
| Level 2 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| Level 3 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| Level 4 | 1 (3.0%) | 0 (0.0%) | 1 (6.25%) | - |
| Level 5 | 5 (15.2%) | 5 (29.4%) | 0 (0.0%) | - |
| Gastrointestinal Symptoms n (%) | ||||
| No | 126 (84.6%) | 68 (81.0%) | 58 (89.2%) | |
| Yes | 23 (15.4%) | 16 (19.0%) | 7 (10.8%) | |
| Speed of Response, months | ||||
| Response reported, n (%) | 125 (83.9%) | 74 (88.1%) | 51 (78.5%) | 0.039 |
| Response reported, mean (SD) | 4.5 (4.4) | 4.9 (4.9) | 4.0 (3.5) | <0.001 |
Abbrevations: IVIG: Intravenous immunoglobulin; SCIG: Subcutaneous immunoglobulin; N/n: Number.
p value <0.05 considered statistically significant
Table 5: Patient outcomes by route of administration.
This study evaluated safety, efficacy, and patient-reported outcomes/perception of response using real-world data from a national specialty home infusion pharmacy in the US. Thus, unlike data generated from a particular IVIG or SCIG manufacturer, or single-center provider experiences, the outcomes reflect the use of multiple products in alternate care settings across the US. The use of home infusion services is continuing to increase, in part because of the growing use of SCIG and the availability of home health nursing. This study demonstrated that SCIG is a viable option or an alternative route of administration for patients with PIDD. SCIG was shown to be safe and effective and demonstrated advantages in patient-reported outcomes compared to IVIG, which is consistent with previously reported data [2,7,16,17].
In assessing real-world safety outcomes between IVIG and SCIG therapies, higher rates of local adverse reactions were observed in patients receiving SCIG, while higher rates of systemic adverse reactions were noted with IVIG. These findings are consistent with those in relevant literature, where few systemic adverse reactions were reported during SCIG infusions, indicating a favorable safety profile compared to IVIG [17,18]. The most common systemic adverse reactions of IVIG infusions were headache, fever, fatigue, vomiting, and chills, which often occur during or within 48 hours of IVIG infusion [2,6-14]. These systemic adverse reactions may be related to the rapid increase in serum IG concentration when patients are given IVIG. Administration of SCIG results in slower absorption with significantly lower peak serum IG levels occurring 2 to 4 days post infusion, which could be a contributing factor to the less frequent occurrence of systemic reactions following infusion of SCIG [2,6-9,14].
Survival of patients with PIDD has increased since the introduction of IG replacement therapy, and the efficacy of IVIG and SCIG in preventing serious bacterial infections is well established [2]. The efficacy data in this study revealed that patients on SCIG had lower overall rates of hospital visits when compared to patients on IVIG. SCIG administered weekly showed comparable efficacy to IVIG in preventing other infections, and is effective in preventing hospitalizations due to infection, and minimizing days missed from work/school and days on antibiotics [2]. Patients on SCIG had lower rates of infections overall when compared to patients on IVIG. Overall, clinical trials of SCIG administered weekly have shown comparable efficacy compared to IVIG in preventing serious bacterial infections in patients with antibody deficiencies [14].
Numerous studies have demonstrated the acceptability of SCIG, as well as its improvements in overall QoL measures [2,16-18]. Prior studies have used standardized validated QoL surveys before starting SCIG and again after 6-12 months [2,18]. Patients on SCIG reported improvements in their feeling of general well-being when compared to IVIG [2,17-21]. Due to the retrospective nature of the study, patient-reported data was not collected from all patients. However, the data collected demonstrated statistical significance with maintenance of normal/moderate levels of energy, less pain, less gastrointestinal symptoms, and faster speed of response with the use of SCIG compared to IVIG.
In our study, the majority of patients (n=124 (83.2%)) did not switch routes of administration. However, converting patients to SCIG from IVIG may be considered advantageous in terms of convenience for patents and their caregivers, while maintaining effectiveness and safety of therapy [22]. Our results demonstrated that SCIG patients had a higher proportion of patients on therapy for a longer period of time (≥ 36 months) versus IVIG.
The limitations of this study include its retrospective nature. Additionally, data retrieval and collection represent possible limitations. In some instances, information was not included in the EMR and/or not obtained in all patients. For this reason, conclusions cannot be imputed or generalized from all study patients. In addition, hospital visits and infections could be attributed to external factors, which cannot be controlled in the real-world setting. Further research is warranted to evaluate the association of patient characteristics and comorbidities with safety and efficacy outcomes.
There have been significant improvements in the treatment of PIDD in the past 60 years, due in large part to IG therapy. It is important to note that both routes of administration have advantages and disadvantages. Various authors have indicated that a patient’s preference is a fundamental factor to take into account, along with clinical criteria, when choosing the route of administration for IG therapy [22]. IVIG may have some limitations in pediatric or elderly patients due to poor venous access, higher expected rates of systemic adverse reactions, and the amount of time it takes for patients to complete infusions. SCIG does not require venous access and is associated with slower release of IG into systemic circulation, creating a depot effect, resulting in more consistent serum steady state levels. The more consistent steady state levels can be associated with lower rates of breakthrough infections and lower rates of wear-off effect [14,17]. Concurrently, the slower release of IG into systemic circulation also results in a lower pharmacokinetic peak concentration in the serum, potentially associated with lower incidence of systemic adverse reactions. In summary, both therapies can be administered at home. However, because of differences in safety profile, route of administration and reduction in the rate of infections, healthcare providers can tailor a therapeutic regimen to suit a patient ’ s lifestyle and medical condition.
In conclusion, in a real-world, home infusion settings, SCIG infusions are safe, efficacious, and well tolerated compared to IVIG and provide PIDD patients with an alternative option for therapy. This study revealed that SCIG demonstrated a beneficial profile in tolerability and efficacy compared to IVIG. Notably, hospital visits and infection rates were significantly reduced in patients receiving SCIG. The overall findings of this study contribute to growing evidence demonstrating the acceptability of SCIG in adult and pediatric patients with PIDD.
This project was generously supported by a grant from CSL Behring, Inc. We also acknowledge the support of Shireen Dunwoody of Dunwoody Consulting for medical writing assistance for the protocol, data interpretation and review, and assistance with development, review, and revisions of the manuscript.
Citation: Geng B, Piracha F, Rashid N, Rigas M (2020) Intravenous versus Subcutaneous Immunoglobulin in Primary Immunodeficiency: Real- World Evaluation of Safety, Efficacy, and Patient Perceptions J Clin Cell Immunol. 11:589. doi: 10.35248/2155-9899.20.11:589.
Received: 15-Apr-2020 Accepted: 29-Apr-2020 Published: 06-May-2020 , DOI: 10.35248/2155-9899.20.11.589
Copyright: © 2020 Geng B et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This project was generously supported by a grant from CSL Behring, Inc. We also acknowledge the support of Shireen Dunwoody of Dunwoody Consulting for medical writing assistance for the protocol, data interpretation and review, and assistance with development, review, and revisions of the manuscript.