Entomology, Ornithology & Herpetology: Current Research
Open Access
ISSN: 2161-0983
ISSN: 2161-0983
Research Article - (2020)Volume 9, Issue 1
Environmental pollutants such as e.g. phosphates, sulphates, are a serious problem that influences the structure and functioning of ecosystems. The levels of these pollutants were measured in soil, plant, insect, and water samples from field site. Monitoring of possible impact of pollutants concentration was assessed using parameters such as protein carbonyls amount, levels of antioxidant enzymatic system. The potential integration between monitoring and bio monitoring systems is to ensure impacts of environmental contamination on living organisms. The results emphasize that concentration of protein carbonyls in the brain, thoracic muscles and gut of Aiolopus thalasinus (Grasshopper) in the field between each other 6.3, 6.87, and 4.47 fold respectively to each other. The levels of protein carbonyls significantly increase in the field samples gut, brain, thorax 2.22 fold, 1.26 fold and 0.74 fold respectively to each other. Otherwise, the activities of antioxidant enzymes such as CAT, and APOX were significantly affected by the environmental pollutants. Specific pollution resulting from the activity of the environmental pollutants caused comparable adverse effects in the brain, gut and thorax of grasshopper. Therefore, the success of using biochemical parameters as a bio monitoring tools of environmental pollutants levels was discussed.
Aiolopus thalasinus; Grasshopper; Protein carbonyls; Environmental pollutants
Biological tissues require oxygen to meet their energetic demands. However, the consumption of oxygen also results in the generation of free radicals that may have damaging effects on cells. The brain is particularly vulnerable to the effects of reactive oxygen species (ROS) due to its high demand for oxygen, and its abundance of highly peroxidisable substrates. Oxidative stress which is defined as the potential of oxygen free radicals and other reactive oxygen species (ROS) is to damage tissues and cellular components. Oxidative stress is caused by an imbalance in the redox state of the cell [1], either by overproduction of reactive oxygen species, or by dysfunction of the antioxidant systems [2,3]. Environmental pollution is considered as a serious problem, both in developed and developing countries, so environmental pollution has become an issue of serious international concern [4]. Diverse protective systems must exist to enable adaptation to oxidative environments. Oxidative stress (OS) results when production of (ROS) exceeds the capacity of cellular antioxidant defenses to remove these toxic species.
There are three major ROS that are of physiological significance are superoxide anion (O2 ‾), hydroxyl radical (OH) and hydrogen peroxide (H2O2) [5,6]. Many environmental pollutants engage signaling pathway that is activated in response to OS. The same sequences of events are also associated with the etiology and early pathology of many chronic diseases. Investigations of oxidative responses in different in vivo models suggest that, in complex organisms such as mammals, organs and tissues contain distinct antioxidant systems, and this may form the basis for differential susceptibility to environmental toxic agents. Thus, understanding the pathways leading to the induction of antioxidant responses will enable development of strategies to protect against oxidative damage [7]. The balance between prooxidant endogenous and exogenous factors (environmental pollutants) and antioxidant defenses (enzymatic and nonenzymatic) in biological systems can be used to assess toxic effects under stressful environmental conditions, especially damage induced by different classes of environmental pollutants [6,8].
Toxic metals (lead, cadmium, mercury and arsenic) are widely found in our environment. Humans are exposed to these metals from numerous sources, including contaminated air, water, soil and food [9]. Highly reactive molecules called free radicals can cause tissue damage by reacting with polyunsaturated fatty acids in cellular membranes, nucleotides in DNA, and critical sulfhydryl bonds in proteins. Free radicals can originate endogenously from normal metabolic reactions or exogenously as components of tobacco smoke and air pollutants and indirectly through the metabolism of certain solvents, drugs, and pesticides as well as through exposure to radiation [10].
Defenses against free radical damage include tocopherol (vitamin E), ascorbic acid (vitamin C), beta-carotene, glutathione, uric acid, bilirubin, and several metalloenzymes including glutathione peroxidase (selenium), catalase (iron), and superoxide dismutase (copper, zinc, manganese) and proteins such as ceruloplasmin (copper). The extent of tissue damage is the result of the imbalance between the free radicals generated and the antioxidant protective defense system. Several dietary micronutrients contribute greatly to the protective system [11]. Insects such grasshopper can be seen, as sensitive to environmental changes. They can be considered as an interesting subject of Eco toxicological research, and a bio monitor of environmental pollutant, including heavy metals [12]. The present work was conducted to integrate between monitoring and bio monitoring to assess the impacts of environmental pollutants on living organisms by:
a) Measuring environmental pollutant concentration (phosphate and sulphate) in soil, water, plant and insect.
b) Evaluate macromolecules oxidative damage, protein carbonyls level.
c) Evaluate antioxidant enzymatic level such as (APOX, CAT and PC) in gut, brain and thorax of female grasshopper.
Life cycle of grasshopper which collected from field and work on it in lab
The grasshopper is a flying animal belonging to order Orthoptera and class Insecta. About 11,000 species exist. They are herbivorous and commonly seen in autumn; a few appear in summer and spring. During mating the male grasshopper deposits sperm into the female's vagina, which finds its way to the eggs through canals known as micropyles. An adult grasshopper goes through the stages egg, nymph and adult, and has a lifespan of approximately one year (Figure 1).
Figure 1. Life cycle of Grasshopper.
Characterization of field
Geographic Information System (GIS) methodology used to determine the location of the field. It is a computer system designed to capture, store, manipulate, analyze, manage and display all kinds of spatial or geographical data [13]. It is enhancing our ability to study and understand the large-scale spatial structure and dynamics of insect populations, as influenced by heterogeneous environments. These tools can only be fully exploited when used in conjunction with monitoring and research techniques such as trapping that provide knowledge of an insect’s biology and ecology [14]. GIS was used to create buffer area and determine the field [15] (Figure 2).
Figure 2. Map showing collection site.
Sample collection
Insect collection: The samples collected from Hawd El-Akhmas, Cairo university campus, (Latitude 30°1′48.76″N) (longitude 31°11′23.07″) we collect the samples in autumn by sweep method [16], the killing glass jar used contain acetone or ethyl acetate, the plastic can melt and insects can get stuck in it [16],insects collected in three different time to ensure that is not casual case, insects were dissected to isolate samples tissue like brain, thorax and gut for further analysis and were stored at -20°C until use.
Plant collection: Plant leaves Collected for detection of environmental pollutants as it may be a link to exposure in humans which may pose a health risk [17]. All samples in collection were free of soil and represent an area of the field by walking in a zig-zag pattern over the area. Store specimens in a dry place. Freezing your dried specimens for 2 weeks will kill insects if they attack specimens [18].
Soil and water collection: Soil samples also collected by zigzag method from the surface. Not sample soil shortly after fertilizer or manure applications, or when the soil is excessively wet or dry to get accurate results. And finally, Irrigation water was collected from canal which plants was irrigate and put in jars as the accumulation of heavy metals in the soil can be cause by waste water irrigation [19]. Then all samples stored at -20°C until use.
Experimental design
Determination of SO42− and PO43-concentration in soil, plant, insect and water samples: Soil, insect and plant samples were digested according to Moor et al., [20]. Briefly, 0.5 g of samples pre-digested with 12 mL 37% HCl: 65% HNO3 (3:1) mixture for 24 h at room temperature. Then, mixture solution of 2.5 mL of 37% HCl and 2.5 mL of 30% H2O2 were added to complete the digestion. Sulphates and phosphates were analyzed in compliance with the method of Radojevic and Bashkin [21]. Absorbance was measured at 420 nm using a UV/Vis Jenway-7305 spectrophotometer (Bibby Scientific Limited, Staffordshire, UK). The concentrations SO42− and PO4 3− in the soil, plant, and water samples were measured in three replicates.
Protein carbonyls assay: We used the procedure from Levine et al. [22] for the protein carbonyls assay, with the below-described modifications. After tissue isolation, samples were homogenized in 5 mL ice-cold phosphate buffer (60 mL of 50 mM phosphate buffer, 10 mL of 0.1% Triton X-100, 5 mL of 0.05 mM CaCl2; then completed to 100 mL with distilled water after adjusting pH to 7.0 with 2M HCl or NaOH). After homogenization (mortar, 10 strokes/30 seconds), the samples were centrifuged at 2000 ×g for 10 min at 4°C. For each tissue extract, an 800 μL aliquot of the supernatant was transferred to a clean microtube with 200 μL of 10 mM 2, 4-dinitrophenyl hydrazine (DNPH) prepared in 2 M HCl. The samples were incubated for 30 minutes at room temperature, precipitated with 10% Tricholoroacetic acid (TCA), and left for 10 min at 4°C. The samples were centrifuged at 5000 ×g for 7 min at 4°C. The pellet was washed four times with an ethanol/ethyl acetate (1:1) mixture, and redissolved in 1 mL of sodium phosphate buffer (60 mL of 150 mM phosphate buffer, 30 mL of 3% sodium dodecyl sulphate, adjusted to a final volume of 100 mL with distilled water after adjusting the pH to 6.8 with 2M HCl or NaOH). Insoluble material was removed by centrifugation at 2000 xg for 1 min. Finally, the absorbance was measured at 366 nm, and the rate of protein carbonyls concentration was expressed as OD/mg protein. Blank was similarly prepared and treated as above except for DNPH that was not added to the sample.
Antioxidant enzyme activity assays
Insects possess antioxidant enzymes that are detectable with oxidants affect. Antioxidant enzymes used:
Catalase (CAT): The activity of catalase (CAT) was assessed in compliance with the method of Aebi [23]. The reaction mixture contained 0.9 mL of a potassium phosphate buffer (50 mM, pH 7.0), 60 μL of the supernatant and 40 μL of freshly prepared H2O2 (10 mM). The change in absorbance was measured at 240 nm over a period of 1 min. The CAT activity was expressed as OD/μg protein/min.
Ascorbate peroxidase (APOX): The activity of ascorbate peroxidase (APOX) was determined according to Asada [24]. The reaction mixture containing 0.9 mL of phosphate buffer (100 mM, pH 7.0) containing 0.1 mM EDTA, 0.3 mM ascorbic acid, 0.06 mM H2O2 and 0.1 mL of the enzyme extract from each sample tissue. Distilled water instead of enzyme extract served as the controls. The change in absorbance was recorded using a spectrophotometer at 290 nm over a period of 30 s after the addition of H2O2. The APOX activity was expressed as OD/μg protein/min.
Determination of SO42− and PO43-concentration in soil, plant, water and insect samples
The results show the concentration of the Sulphate in soil, plant, water and insect in the control site. The highest concentration of Sulphate is present in the soil (0.001212943 mean). The concentration of plant and water has no significance difference between them (mean 0.000889127 and 0.00075922). The insect have (mean 3.8306E-05) (Figure 3).
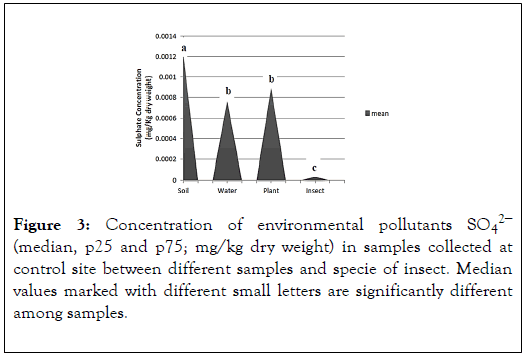
Figure 3. Concentration of environmental pollutants SO42− (median, p25 and p75; mg/kg dry weight) in samples collected at control site between different samples and specie of insect. Median values marked with different small letters are significantly different among samples.
The result shows the levels of environmental pollutants (sulphate) in control site. By comparing the results with the control values estimated by EPA of control site, which is 1.4 in plant, 2.5 in water and 8.8 in soil. We also compared the results of insect with the negative control value of grasshopper in lab which is 0.3 [6]. We observed that the levels are within normal levels but it does not ensure that it has no effect on organisms. The results show the concentration of the phosphate in soil, plant, water and insect in the control site. The highest concentration of sulphate is present in the soil and water have no significance difference between them (mean 0.01705and 0.017766667). The concentrations of plant and insect have no significance difference between them (mean 0.001891667 and 0.000329167) (Figure 4).
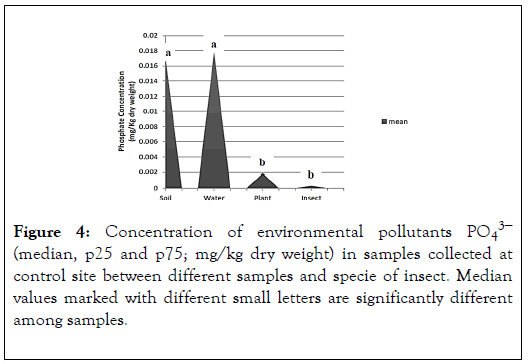
Figure 4. Concentration of environmental pollutants PO43− (median, p25 and p75; mg/kg dry weight) in samples collected at control site between different samples and specie of insect. Median values marked with different small letters are significantly different among samples.
The result shows the levels of environmental pollutants (phosphate) in control site. By comparing the results with the control values estimated by EPA of control site, which is 13 in plant, 1.8 in water and 16 in soil. We also compared the results of insect with the negative control value of grasshopper in lab which is 0.49 [6]. We observed that the levels are within normal levels but it does not ensure that it has no effect on organisms.
Determination of CAT (Catalase) and APOX (ascorbate peroxidase) in Grasshopper
The result shows the activity of catalase enzyme. The highest result is in Grasshopper’s Brain in field (mean 5982) (Figure 5).
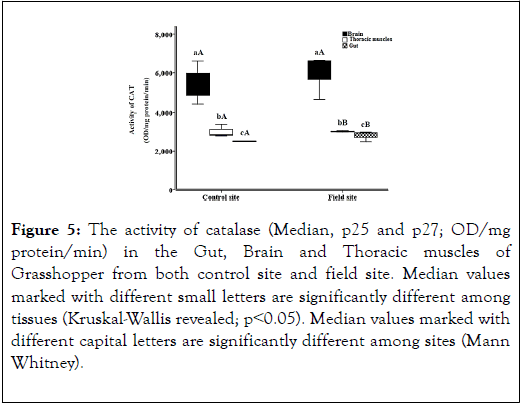
Figure 5. The activity of catalase (Median, p25 and p27; OD/mg protein/min) in the Gut, Brain and Thoracic muscles of Grasshopper from both control site and field site. Median values marked with different small letters are significantly different among tissues (Kruskal-Wallis revealed; p˂0.05). Median values marked with different capital letters are significantly different among sites (Mann Whitney).
The result shows the levels of antioxidant enzyme (catalase) in control site (negative control in lab) and in field site (Hawd El- Akhmas). By comparing the results of grasshopper in Brain, Thoracic muscle and Gut, which is 5982, 3011 and 2798 with the negative control of grasshopper in lab, which is 5466, 3002 and 2516. We observed that the levels are slightly elevated than the negative control greatly. That indicates the increase in the enzyme to be able to deal with oxidants elevation in insects by its oxidation. The result shows the activity of ascorbate peroxidase enzyme. The highest result is in Grasshopper’s Brain in field (mean 3995) (Figure 6).
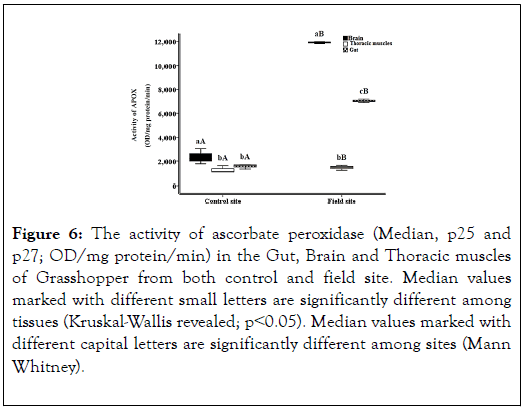
Figure 6. The activity of ascorbate peroxidase (Median, p25 and p27; OD/mg protein/min) in the Gut, Brain and Thoracic muscles of Grasshopper from both control and field site. Median values marked with different small letters are significantly different among tissues (Kruskal-Wallis revealed; p˂0.05). Median values marked with different capital letters are significantly different among sites (Mann Whitney).
The result shows the levels of antioxidant enzyme (ascorbate peroxidase) in control and field site. By comparing the results of grasshopper in Brain, Thoracic muscles and Gut, which is 3995, 1458 and 2391 with the negative control of grasshopper in lab, which is 2342, 1269 and 1566. We observed that the levels are slightly elevated than the negative control greatly. That indicates the increase in the enzyme to be able to deal with oxidants elevation in insects by its oxidation.
Determination of protein carbonyls in Grasshopper
The result shows the amount of protein carbonyls enzyme. The highest result is in Grasshopper’s Gut in control (mean 9.93) (Figure 7). The result shows the level of damage (protein carbonyls) in control and field site. By comparing the results of grasshopper in Brain, Thoracic muscles and Gut; which is 6.87, 6.3 and 4.47 in control site with the negative control of grasshopper in lab, which is 8.71, 4.69 and 9.93. We observed that the levels are slightly elevated than the negative control greatly. That indicates the increase in the damage due to the increase of the oxidants.
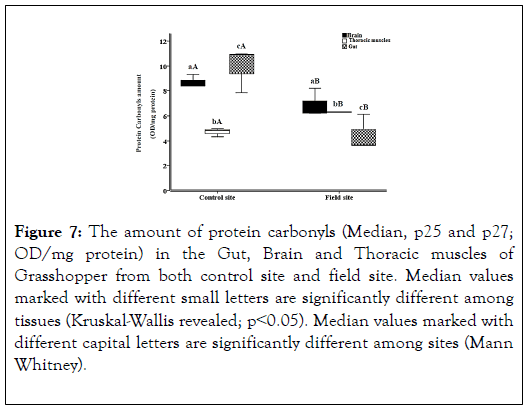
Figure 7. The amount of protein carbonyls (Median, p25 and p27; OD/mg protein) in the Gut, Brain and Thoracic muscles of Grasshopper from both control site and field site. Median values marked with different small letters are significantly different among tissues (Kruskal-Wallis revealed; p˂0.05). Median values marked with different capital letters are significantly different among sites (Mann Whitney).
We performed bio monitoring on different levels of life as soil, plant, water and even organisms (as insects) detected the levels of contamination and its effect on our planet in these levels. From our studies of the levels of re oxidant enzymes (CAT, PC, APOX), we viewed the effect of contamination on re-oxidant enzymes, that lead to inhibition of these enzymes by our compare to its control. These results could be the next generation of finding a way to prevent the oxidative stress done by contamination which is the result of many diseases including cancer, which is the second lethal disease after heart attaches. From our studies, we proved the advantage of the use of bio monitor over traditional monitoring of air (which only detects the level of contamination without any information of its effect on our planet and on life itself for all organisms) on the basis of both economics and health departments. Recent studies indicate that transition metals act as catalysts in the oxidative reactions of biological macromolecules therefore the toxicities associated with these metals might be due to oxidative tissue damage [25].
Protein carbonylation: Protein carbonylation is one of the negative effects revealed during the process of oxidative stress and loss of antioxidant enzyme. The major site for protein carbonylation is the adipose tissue and it is defined as the appearance of carbonyl group in proteins due to direct oxidation of side chains of lysine, arginine, proline, and threonine residues that make it more hydrophobic and resist to proteolysis [26]. Carbonyl stress, characterized by accumulation of reactive carbonylated species as high molecular weight carbonyl groups (e.g., aldehydes, ketones, and lactams) for lipids, nucleic acids and proteins, and their reactivity towards nucleophilic substrates, results in biomolecule malfunctions and increased toxicity and can finally lead to apoptotic cell death. Oxidative decomposition of polyunsaturated fatty acids initiates chain reactions lead to the formation of a variety of carbonyl species (three to nine carbons in length), the most reactive and cytotoxic being α,β-unsaturated aldehydes (4-hydroxy-trans-2-nonenal and acrolein), di-aldehydes (malondialdehyde and glyoxal), and keto-aldehydes (4-oxo-trans-2nonenal). Metal-catalyzed oxidation (MCO) was first identified as a source of protein-bound carbonyls (E. R. (1986) Trends Biochem. Sci. 11, 11-12) MCO results from the Fenton reaction, when transition metal ions are reduced in the presence of hydrogen peroxide with the generation of highly reactive hydroxyl radicals. These hydroxyl radicals oxidize amino acid side chains or cleave the protein backbone, leading to numerous modifications including reactive carbonyls.
In the present work, part of biomonitoring program designed to evaluate the biochemical changes in insect, grasshopper, collected from Hwad El-Akhmas. The concentration of soil, plant, and water pollutants such as phosphate (PO43-), and sulphate (SO42-) were generally within national (Egyptian limits) and international standard limits [27]. The biomonitoring programs involve bioaccumulation, biochemical alterations, morphological and behavior observation, population- and community-level approaches. The applications of biomonitoring programs are evaluation of metal concentration, toxicology prediction and researches on toxicological mechanism, toxicological prediction and bioremediation [28,29]. Toxicological mechanism included detecting the action mechanism through interaction between pollutants and biological macromolecules such as proteins, enzymes and nucleic acid [30]. Proper quality management system (QA) should be applied to make sure that biomonitoring studies are scientifically valid, comparable and robust to evaluate the relationship between environmental pollutants and its toxicological effect in living organism. The concentrations of environmental pollutants, PO43- and SO42- were in the normal range in soil, plant, and water samples collected from control site compared to the control values estimated by EPA of control site, which is 1.4 in plant, 2.5 in water and 8.8 in soil (Figures 3 and 4). Phosphorus species were considered the principal carriers of trace elements in soils. The phosphate industry possesses a serious soil pollution hazard, with deposited contaminants being potentially hazardous to plants and groundwater [9].
Environmental pollutants lead to imbalance between ROS levels and antioxidants of exposed organisms and cause oxidative stress such as hydrofluoric acid fumes HF, SO2, NO2, PM10, waste products of fertilizer industry such as PO43-, SO42-, dust, and heavy metals can increase production of ROS in cells of exposed organisms and caused oxidative stress. The pollutants which used in industry and agriculture include metals, metalloids, and numerous other organic compounds, lead to increase the production of reactive oxygen species (ROS) in the cells of individuals exposed to them, and therefore caused oxidative stress with all the adverse consequences for organisms [6,8,31-34]. Ahmad [35] and Chaitanya et al. [36] approved that, the pollution of environmental components (air, soil, water and plant) was considered as a non-biological factor of oxidative stress in invertebrates. In the present work, the level of stress was evaluated indirectly, by the measurement of oxidative damage of macromolecules: proteins, and as well as measurement of enzymatic antioxidant (CAT and APOX) response in gut, brain, thorax in grasshopper. This was done in accordance with generally accepted knowledge [37,38]. Molecular mechanisms causing DNA damage may include: activation of nucleases, direct reaction of free radicals such as hydroxyl radicals (•OH) with the DNA, or breaks resulting from free radical reaction of deoxyribose residues, invariably possess blocked termini [39,40]. Moreover, DNA damage can involve: formation of a basic site that finally leads to strand breaks [39,41,42]; modifications and degradations of nitrogenous bases; damage to sugar moiety; formation of DNA-DNA and DNA-protein cross links, and damage to the repairing system of DNA [43,44].
It was proved that metals such as Zn caused DNA damage in cells isolated from the gut, brain, thorax in grasshopper but this effect was not proportional to the metal dose [45]. Copper, another essential trace element, can induce oxidative damage to macromolecules such as DNA, proteins and lipids [46]. In the presence of the reducing agents, Cu can catalyze the production of ROS, such as superoxide anion radical (O2 •-), hydrogen peroxide (H2O2) and •OH, through Fenton and Haber-Weiss reactions. Previous studies showed that DNA breaks are caused by a site-specific reaction of Cu ions, both in vitro and in vivo [47]. Also, Lionetto et al., [48] found that inhibition of enzymatic activity as a result of Cu toxicity. Another study revealed the sensitivity of acetylcholinesterase to organophosphorus insecticide. These insights open new perspective for the future use of this biomarker in environmental health monitoring [49]. Yousef et al. [50] found that the genotoxicity of heavy metals, cadmium and lead, in grasshopper was very high. Hence, this may reflect the role played by grasshoppers as a valuable bio indicator of environmental genotoxic pollutants. Joseph [51] hypothesized that genotoxic effect of Cd may also result from generation of ROS, and lead to oxidative stress that is associated with generation of 7,8-dihydro-8-oxoguanine (8oxoGua) commonly used to monitor DNA damage [46]. Some studies proved that inhalation of SO2 caused a significant increase in DNA damage in various organs of both males and females of albino mice which could cause mutation, cancer, and other diseases related to the DNA damage [52]. Severe intensification of oxidative stress and the increase in DNA damage – occurring due to PM exposure – were described [53-55].
The present result revealed the spectacular elevation of protein carbonyls in tissues of insects collected at control site compared to the negative control of grasshopper in lab. Some insects can eliminate heavy metals from their bodies through ecdysis and metamorphosis [56], and through excretion in the feces during grasshopper development [57]. The obtained results revealed the information that proteins are important targets of free radical attack in the cells [58], and thus, the antioxidant defense, cellular function, and finally organism survival can be impaired. ROS are known to convert amino groups of proteins and thereby, change protein structure and function. The oxidative stress increased the number of modified carbonyl groups correlates with protein damage [1]. Also, ROS can cause fragmentation of the peptide chain, alteration of electrical charge of proteins, cross-linking of proteins and oxidation of specific amino acids and therefore lead to increase susceptibility to proteolysis by degradation of specific proteases. The oxidation of proteins leads physiologically to disruption of conformation and vital functions of protein molecules, including enzymes, and other regulatory functions of the cell [44]. There are key antioxidant enzymes responsible for scavenging of oxygen radicals. However, environmental pollutants such as sulphate and phosphate increase the production of reactive oxygen species (ROS), and, directly or indirectly, cause oxidative damage by inhibiting activity of antioxidant enzymes. Previous studies suggested that inhibition of antioxidant enzymes activity occurred due to decrease expression level of antioxidant enzymes.
Integration between monitoring and biomonitoring systems to assess impacts of environmental pollutants enhances us not only to detect concentration of these pollutants in the ecosystem, but also to evaluate their effect on living organisms. By using in coccinella undecimpuctata and P.alceae as bio monitor for the contamination level in Hud El-AKmas, we found that antioxidant enzymes (APOX, CAT and PC) affected (elevating) indicating that there is pollution however the results of phosphate and sulphate test are within normal range. This is the discrimination of integrating between monitoring and biomonitoring. We can ascertain from the contamination level exactly. Thus, we can keep the health of human and environment as well keeping the balance between ecological systems and biodiversity help our country in attaining the sustainable development goals, Egypt ’ s Vision 2030 and supporting the economy by our seeking to convert our research to startup. After ensuring that field is a control site suitable for building our startup which is FOTM (From Organic To Mash), we exploit our dealing with various insects and flies and our passion to achieve 3rd generation universities goals which focus on graduation of entrepreneurs who are able to solve our country problems.
Citation: Abdelfattah EA (2020) Integration Between Monitoring and Bio-Monitoring Systems to Assessment the Impacts of Normal Levels of Environmental Pollutants. Entomol Ornithol Herpetol. 9:221. DOI: 10.35248/2161-0983.20.9.221.
Received: 14-Jan-2020 Accepted: 03-Feb-2020 Published: 10-Feb-2020 , DOI: 10.35248/2161-0983.20.9.221
Copyright: © 2020 Abdelfattah EA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.