Journal of Oceanography and Marine Research
Open Access
ISSN: 2572-3103
ISSN: 2572-3103
Research Article - (2020)Volume 8, Issue 2
Total Volatile Acids (TVA) and Total Volatile Bases (TVB) are principal spoilage indicators monitored to determine the degradation of the fish muscle tissue. This study seeks to monitor the TVA and TVB concentrations using a Flow Injection Analysis – Gas Diffusion (FIA-GD) method on Scomberomorus brasiliensis (Carite). Samples were purchased from Supermarket operators (Single and Chain); fishmongers (who will ply their trade in the mornings and evenings); and fresh fish muscle tissue stored at -15°C, 1°C, and 29°C over a 12-month period. All the fish samples in this study, except twelve samples stored at 29°C, should be considered highly edible (TVA < 220 ppm and 200 ppm < TVB < 600 ppm). Analysis of variance tests (α=0.05) on the data indicated that there are significant differences among the values measured for the other locations or storage conditions. Samples from Single- and Chain- operator supermarkets were about the same levels of TVA and TVB, but these are different from those observed for the highway fishmongers' samples (AM and PM). Higher TVA and TVB concentrations observed at higher storage temperature (29°C).
Scomberomorus brasiliensis (Carite); Total volatile acid; Total volatile base; Flow injection analysis – gas diffusion (FIA-GD) method; Supermarket operators; Highway fishmongers and storage temperatures
In the freshness determination of fish and fish products, general methods have to be developed which would be applicable for all species of fish kept under different storage conditions. These methods should be rapid, easy, and should correlate well with sensory evaluation. One such procedure is the official method of analysis of the Total Volatile Acids and Bases content which measures the lower members of the fatty acid series {free fatty acids (FFA) with less than six carbon atoms} including methanoic (HCOOH), ethanoic (CH3COOH), propanoic (CH3CH2COOH), and butanoic acids (CH3(CH2)2COOH) [1,2]. A natural occurring post-mortem feature in fish is lipid hydrolysis which initiates the formation of free fatty acids. FFA (measured by TVA assay) and glycerol are by-products of the deterioration of the fish muscle tissue by endogenous and/or bacterial lipolytic enzymes. Free fatty acids are essentially absent in the fish fat tissue but can be generated after the fish dies [3]. Therefore, lipid hydrolysis of chill-stored fish is of negligible importance, when substantial FFA concentrations may be developed during storage of the whole fish, particularly at elevated temperatures [4].
Traditionally, the Total Volatile Acid content is determined by two methods, (i) the titrimetric ethanoic acid method, where the values are calculated through titration against sodium hydroxide and (ii) the enzymatic/ethanoic acid procedure which requires the use of diamine oxidase [5]. These methods, are not always applicable to the routine testing of large numbers of fish samples. Random errors may be due to air drafts and uneven heating [6]. However, the enzymatic method is quicker, more specific, and lends itself to the processing of large numbers of samples, but it is very costly.
The concentration of the total volatile bases (TVB) present in the fish muscle tissue is determined by the traditional semi-micro distillation procedure as outlined by Williams [7].
The use of Flow Injection Analysis (FIA) with Gas Diffusion circumvents many of these problems (Figure 1). Gas Diffusion involves the transport of the volatile analyte of interest Total Volatile Acids and Bases, (TVA; TVB), from a donor stream across a permeable barrier into a recipient stream. The analyte may be detected directly by electrochemical means or indirectly using a colorimetric reagent [5]. The pH of the donor stream is designed to keep the analyte (fish muscle extract) in its acidic form so that when it diffuses across the membrane, there is a pH change in the recipient stream. In the present study, these pH changes are monitored through the use of an indicator (methyl red). Ethanoic acid was used as the standard in this procedure for TVA and TVB, phenol red as the indicator and ammonium chloride as the standard.
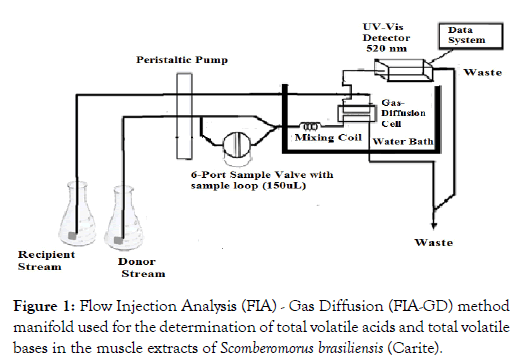
Figure 1: Flow Injection Analysis (FIA) - Gas Diffusion (FIA-GD) method manifold used for the determination of total volatile acids and total volatile bases in the muscle extracts of Scomberomorus brasiliensis (Carite).
Advantages of using a Flow Injection Analysis – Gas Diffusion method are the method is relatively rapid (throughput=6 samples/hr), reproducible, sensitive, economical and very efficient separation of the analyte from sample components that may otherwise interfere with the detection method. This study seeks to monitor the variations of the Total Volatile Acids and Bases content in Scomberomorus brasiliensis (Carite) samples purchased from Supermarket operators (Single and Chain); Highway fishmongers (who will ply their trade in the mornings and evenings); and fresh fish muscle tissue stored at -15°C, 1°C, and 29°C.
Fish source
Whole Scomberomorus brasiliensis (Carite) fish samples were purchased from Supermarket operators (Single and Chain); Highway fishmongers (who will ply their trade in the morning and evening periods). Fresh fish muscle tissue stored at -15°C, 1°C, and 29°C was also incorporated in this 12-months study.
Three whole Scomberomorus brasiliensis (Carite) fish samples from the different locations were iced and taken to the laboratory. Each fresh sample was in an early post-rigor condition (12 hours after netting them). All the fish samples measured between 30-40 cm. The temperatures of these coolers were -15°C, 1°C, and 29°C. The samples brought from the supermarkets or the fishmongers were stored at 10 ± 2°C during their trip back to the laboratory.
Flow Injection Analysis (FIA) - Gas Diffusion (FIA-GD) method
A gas diffusion FIA method was used to determine total volatile acids and bases (Figure 1). The apparatus consisted of a low pulse peristaltic pump (Gilson Minipuls 2) equipped with standard high density propylethylene (HDPE) pump tubing (2.00 mm i.d.), all other tubing and fitting being Teflon® (0.5 mm i.d.). The system consisted of a six-port rotary injection valve (Model 5020, Rheodyne Inc.); a low volume gas diffusion cell, equipped with a Teflon® membrane (150 mm, 5 μm pore size, Cole-Palmar) and a UV/VIS spectrophotometer (Philips, PU 8625), fitted with a flow cell (10 μL), and connected to a dual pen strip chart recorder (Kipp and Zonen BD41).
Fish muscle tissue (10 g), was blended at high speed (for 2 minutes) with sulphuric acid (0.01 mol L-1, 90 mL) and centrifuged for 10 min. at 2976 g. The supernatant (150 μL) was injected directly into the donor stream of the FIA manifold (Figure 2). This was either sulphuric acid (0.5 mol L-1), for TVA determinations or sodium hydroxide (0.1 mol L-1), for TVB determinations. The recipient stream for TVA measurements was a solution of methyl red (12 ppm, pH 5.0) in sodium chloride (1 mmol L-1). For TVB measurements it was a solution of phenol red (35 ppm, pH 6.0) in sodium chloride (1 mmol L-1). Detection wavelengths were at 520 nm and 555 nm for TVA and TVB determinations respectively. For TVA and TVB, the calibration standards utilized were ethanoic acid (10-100 ppm) and ammonium chloride (10-100 ppm) respectively. Conditions for the determination of Total Volatile Acids and Bases {ppm} in the muscle tissue of Scomberomorus brasiliensis (Carite) purchased from four (4) locations and stored under different conditions using FIA-Gas Diffusion method are given in Table 1.
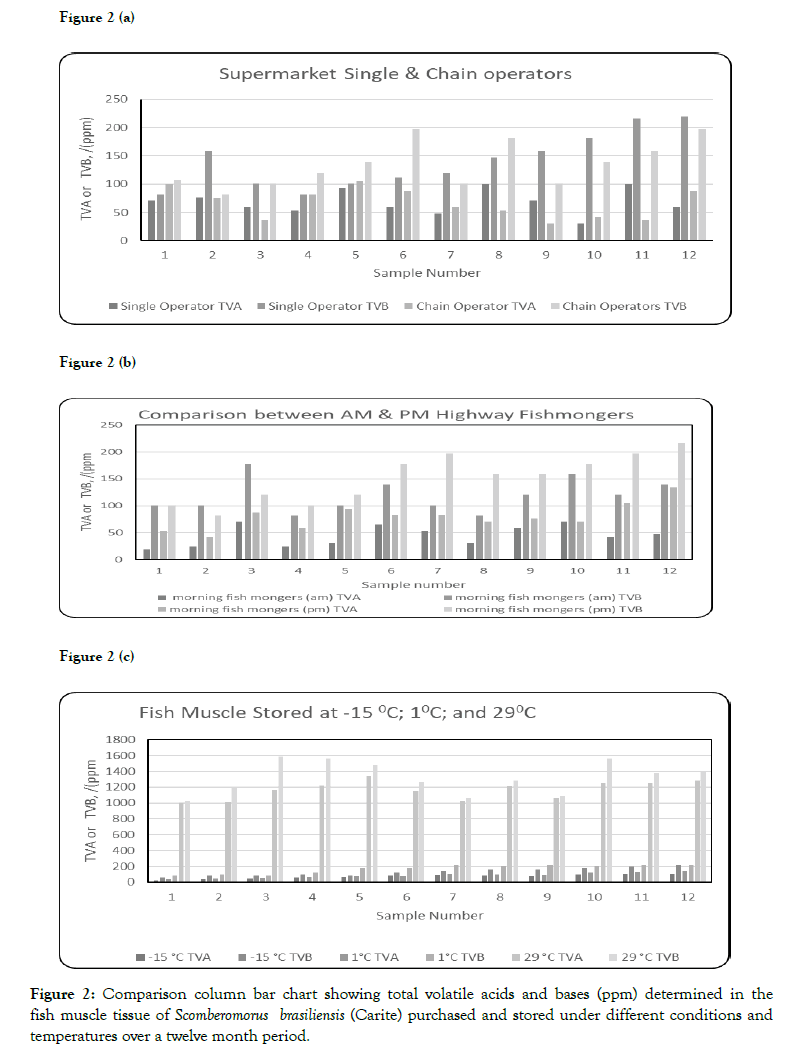
Figure 2: Comparison column bar chart showing total volatile acids and bases (ppm) determined in the fish muscle tissue of Scomberomorus brasiliensis (Carite) purchased and stored under different conditions and temperatures over a twelve month period.
| Reagents | Total Volatile Acids | Total Volatile Bases |
|---|---|---|
| Donor Stream | Sulphuric acid (0.5 mol L-1) |
sodium hydroxide (0.1 mol L-1) |
| sulphuric acid (0.5 mol L-1) |
||
| Recipient Stream | methyl red (12 ppm, pH 5.0) in sodium chloride (1 mmol L-1) | phenol red (35 ppm, pH 6.0) in sodium chloride (1 mmol L-1) |
| Conditions | ||
| Donor Stream | 0.14 mL/min | 0.11 mL/min |
| Sample Stream | 0.15 mL/min | 0.13mL/min |
| Recipient Stream | 0.30 mL/min | 0.25 mL/min |
| Injection Volume (loop size) | 150 µL | 150 µL |
| Mixing coil volume | 150 µL | 150 µL |
| UV/Vis Detector wavelength setting | 520 nm | 555 nm |
| Flow Cell | 10 μL | 10 μL |
| Calibrating standards | Ethanoic Acid (10-100 ppm) | ammonium chloride (10-100 ppm) |
| Water bath temperature | 40 ± 2°C | 40 ± 2°C |
Table 1: Conditions for the determination of total volatile acids {ppm} and total volatile bases {ppm} in the muscle tissue of Scomberomorus brasiliensis (Carite) purchased from four (4) locations and stored under different conditions using FIA-Gas Diffusion method.
The bacterial and enzyme actions, which occur during spoilage, promote the formation of volatile acids, in particular, short-chain fatty acids [1]. By Varga, et al. and McCarthy, et al. criteria, all the fish samples in this study, except the twelve samples stored at 29°C, should be considered highly edible (TVA < 220 ppm and 200 ppm < TVB < 600 ppm) [8,9].
All the TVB values are higher than the corresponding TVA values, a phenomenon also reported by McCarthy et al. in decomposition studies with cod and mackerel [9]. Varga et al. used an experienced test panel and chemical analyses to assess the freshness of cod fillets [8]. They concluded that fish was fresh and highly edible when TVB ≤ 200 ppm, edible when 200 ppm < TVB < 600 ppm, and inedible if the TVB content of the muscle was greater than 600 ppm. McCarthy et al. correlated TVA and TVB values in their study and suggested that upper limits for TVA values corresponding to those suggested by Varga et al. for TVB should be 220 ppm, 450 ppm and 660 ppm for highly edible, edible and inedible fish respectively [8,9].
By Varga's and McCarthy's criteria, all the fish samples in this study, samples stored at 29°C, should be considered highly inedible (TVB >200 ppm, TVA > 220 ppm) [8,9]. Analysis of variance tests (α=0.05) on the data in Table 2, however, indicate that there are real differences among the values measured for the other locations or storage conditions, even though they are all < 200 ppm (except samples stored at 29°C). Samples from single- and chain- operator supermarkets yield essentially the same levels of both TVA and TVB, but these are different from those observed for the highway fishmongers' samples.
| Sample Site | Para-meter | Sample Number | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |||
| Super markets | φ Single | TVA | 70.62 | 76.37 | 59.11 | 53.36 | 93.64 | 59.11 | 47.61 | 99.39 | 70.62 | 30.34 | 99.39 | 59.11 |
| Operators | TVB | 81.6 | 158.5 | 100.8 | 81.6 | 100.74 | 110.83 | 120.1 | 146.96 | 158.51 | 181.6 | 216.2 | 219.3 | |
| π Chain | TVA | 99.39 | 75.54 | 36.1 | 82.13 | 105.14 | 87.88 | 59.11 | 53.36 | 30.34 | 41.85 | 36.09 | 87.88 | |
| Operators | TVB | 107 | 81.6 | 100.8 | 120.06 | 139.29 | 196.97 | 100.8 | 181.6 | 100.83 | 139.28 | 158.51 | 197 | |
| High- | € morning | TVA | 18.84 | 24.59 | 70.62 | 24.59 | 30.34 | 64.87 | 53.35 | 30.34 | 59.11 | 70.62 | 41.85 | 47.61 |
| Way | period | TVB | 100.8 | 100.8 | 177.7 | 81.6 | 100.83 | 139.29 | 100.8 | 81.6 | 120.06 | 158.51 | 120.05 | 139.3 |
| Fish-mongers | evening | TVA | 53.36 | 41.85 | 87.88 | 59.11 | 93.64 | 82.13 | 82.74 | 70.62 | 76.36 | 70.62 | 105.14 | 133.9 |
| period | TVB | 100.8 | 81.6 | 120.1 | 100.83 | 120.06 | 177.74 | 197 | 158.51 | 158.13 | 177.74 | 196.96 | 216.2 | |
| Fresh | -15°C | TVA | 24.59 | 36.1 | 47.61 | 59.11 | 70.62 | 82.13 | 87.88 | 82.13 | 76.36 | 99.39 | 105.14 | 110.9 |
| Fish | TVB | 62.37 | 81.6 | 81.6 | 100.83 | 81.6 | 120.06 | 139.3 | 158.51 | 158.51 | 177.74 | 196.67 | 216.2 | |
| muscle tissue | 1°C | TVA | 36.1 | 47.61 | 53.36 | 64.87 | 76.37 | 76.94 | 105.1 | 99.39 | 93.64 | 122.4 | 128.16 | 139.7 |
| stored | TVB | 81.6 | 100.8 | 81.6 | 120.06 | 177.74 | 177.74 | 216.2 | 205.42 | 213.88 | 203.88 | 213.11 | 212.3 | |
| at (°C) | 29°C | TVA | 1007 | 1015 | 1167 | 1224 | 1339.1 | 1154.2 | 1027 | 1210.5 | 1069.3 | 1254.8 | 1255.1 | 1284 |
| TVB | 1025 | 1201 | 1585 | 1558.5 | 1477.4 | 1269.7 | 1064 | 1287.4 | 1089.7 | 1560.3 | 1377.4 | 1398 | ||
Table 2: Total volatile acids {ppm} determined in the muscle tissue of Scomberomorus brasiliensis (Carite) purchased from four (4) locations and stored under different conditions.
Comparison between the single and chain operators
Comparison between the supermarket operators (Single and Chain) showed that the levels of the TVA (ppm) for the Chain operators had the highest value of 105.14 ppm for sample # 5 compared to the Single operator (99.39 ppm, sample #11). The highest TVB was observed in sample #11 (Single operator). Figure 2A shows the variations in the TVA and TVB between the two supply chains.
The TVA values ranged from 30.34 to 99.39 ppm and 30.34 to 105.14 ppm for the Single and Chain operators respectively. The TVB values ranged from 81.60 to 216.20 ppm and 81.60 to 196.97 ppm for the Single and Chain operators respectively. Factors contributing to the TVA TVB observed in samples obtained from both operators may be due to the overstocking of the freezers in these supermarkets. Also, there may be electricity fluctuations in the freezers, resulting in unregulated temperature variations and the onset of spoilage in the fish muscle tissue.
Comparison between the highway fishmongers (morning period 6:00 am to 12:00 noon, and evening period 12:01 pm to 6:00 pm)
Comparison between the Highway fishmongers (AM and PM) showed the highest TVA occurred on the evening period, sample # 12 (133.91 ppm) and TVB (216.19 ppm). Figure 2B shows the variations in the TVA and TVB between the two fish-mongers morning and evening periods. The TVA values ranged from 18.84 to 70.62 ppm and 41.85 to 133.91 ppm for the Highway fish mongers morning and evening periods respectively. Factors contributing to the TVA and TVB observed in samples obtained from both Highway fish mongers may be due to the freshness of the fish. The fish are purchased in the morning periods directly from the fishmongers, and kept the whole two periods (12 hours) in the sun and exposed to road side conditions. The highest TVA value (133.91 ppm) and TVB (216.19 ppm) observed in evening period is due to rapid onset spoilage. There are no freezers or cold storage facilities present with the Highway fishmongers. The unsold fish are stored in coolers or buckets with ice/ice water (usually between 10°C to 20°C). The displayed fish samples are sprinkled with the same ice water using a recycled plastic bottle and fanned with old newspapers or pieces of cardboard to keep the flies away.
Comparison between the fish muscle tissue stored at -15°C, 1°C, and 29°C
Whole fish bought directly from the fishing boats (Cocorite Fishing Port, Trinidad, W.I.) and stored at -15°C, 1°C, and 29°C.
The ranges (as shown in Table 2) of the TVA were (24.59 to 110.89 ppm), (36.1 to 139.66 ppm), and (1007.33 to 1284.19 ppm) for the fish muscle tissue stored at -15°C; 1°C; and 29°C respectively. TVB were (62.37 to 216.20 ppm), (81.60 to 216.20 ppm), and (1024.6 to 1585.13 ppm) for the fish muscle tissue stored at -15°C, 1°C, and 29°C respectively. Figure 2C shows the comparison variations in the TVA at the three monitored temperatures. The highest range occurred at the storage temperature of 29°C, this is due to changes in the fish muscle tissue’s quality and freshness which depend on the length of storage period and temperature. Fish spoilage occurs rapidly after harvesting, commencing within 12 h at high ambient temperatures (29°C). In accordance with Varga's and McCarthy's criteria {highly edible fish muscle tissue with edible (TVA < 220 ppm and 200 ppm < TVB < 600 ppm)} [8,9]. All the fish samples in this study, stored at 29°C were found to inedible with [TVA] >220 ppm.
A one-way ANOVA test (α=0.05) indicates significant differences among the tissue of fish purchased from the four different locations. There was, however, a marked effect of storage temperature on Total Volatile Acids and Total Volatile Bases content found in the Carite muscle tissue (Table 3). The mean TVA concentration more than twelve times when the storage temperature is increased from -15°C to 29°C, and nine times when the storage temperature is increased from 1°C to 29°C. The mean TVB concentrations were seven times when the storage temperature is increased from -15°C and 1°C to 29°C. Samples from Single- and Chain- operator supermarkets yield essentially the about same levels of TVA, but these are different from those observed for the Highway fishmongers' samples (AM and PM). Higher TVA and TVB concentrations were observed at higher storage temperature (29°C). There was no correlation of TVA and TVB between the Single operators and Chain Operators; or Highway fishmongers (AM and PM). There were TVA and TVB correlations between the following storage temperatures- TVA and TVB at -15°C (0.9142); TVA at -15°C and TVA at 1°C (0.9712); TVA at -15°C and TVB at 1°C (0.9092); TVA at -15°C and TVB at 1°C (0.9619); TVB at -15°C and TVB at 1°C (0.8321).
| Sample Site | Para-meter | Sample Number | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |||
| Super markets | φ Single Operators | TVA | 70.62 | 76.37 | 59.11 | 53.36 | 93.64 | 59.11 | 47.61 | 99.39 | 70.62 | 30.34 | 99.39 | 59.11 |
| TVB | 81.6 | 158.51 | 100.83 | 81.6 | 100.74 | 110.83 | 120.06 | 146.96 | 158.51 | 181.6 | 216.2 | 219.28 | ||
| π Chain | TVA | 99.39 | 75.54 | 36.1 | 82.13 | 105.14 | 87.88 | 59.11 | 53.36 | 30.34 | 41.85 | 36.09 | 87.88 | |
| Operators | TVB | 106.97 | 81.6 | 100.83 | 120.06 | 139.29 | 196.97 | 100.83 | 181.6 | 100.83 | 139.28 | 158.51 | 196.96 | |
| High-Way Fish-mongers |
€ Morning period | TVA | 18.84 | 24.59 | 70.62 | 24.59 | 30.34 | 64.87 | 53.35 | 30.34 | 59.11 | 70.62 | 41.85 | 47.61 |
| TVB | 100.83 | 100.83 | 177.74 | 81.6 | 100.83 | 139.29 | 100.83 | 81.6 | 120.06 | 158.51 | 120.05 | 139.28 | ||
| Evening period | TVA | 53.36 | 41.85 | 87.88 | 59.11 | 93.64 | 82.13 | 82.74 | 70.62 | 76.36 | 70.62 | 105.14 | 133.91 | |
| TVB | 100.83 | 81.6 | 120.06 | 100.83 | 120.06 | 177.74 | 196.96 | 158.51 | 158.13 | 177.74 | 196.96 | 216.19 | ||
| Fresh Fish muscle tissue stored at (°C) |
-15°C | TVA | 24.59 | 36.1 | 47.61 | 59.11 | 70.62 | 82.13 | 87.88 | 82.13 | 76.36 | 99.39 | 105.14 | 110.89 |
| TVB | 62.37 | 81.6 | 81.6 | 100.83 | 81.6 | 120.06 | 139.29 | 158.51 | 158.51 | 177.74 | 196.67 | 216.2 | ||
| 1°C | TVA | 36.1 | 47.61 | 53.36 | 64.87 | 76.37 | 76.94 | 105.14 | 99.39 | 93.64 | 122.4 | 128.16 | 139.66 | |
| TVB | 81.6 | 100.83 | 81.6 | 120.06 | 177.74 | 177.74 | 216.2 | 205.42 | 213.88 | 203.88 | 213.11 | 212.34 | ||
| 29°C | TVA | 1007.3 | 1015.42 | 1166.5 | 1224.03 | 1339.11 | 1154.18 | 1027.45 | 1210.46 | 1069.3 | 1254.83 | 1255.1 | 1284.19 | |
| TVB | 1024.6 | 1200.57 | 1585.13 | 1558.52 | 1477.41 | 1269.69 | 1063.73 | 1287.37 | 1089.73 | 1560.32 | 1377.4 | 1398.21 | ||
φ Single Operators refers to supermarkets with only one outlet.
π Chain Operators refers to supermarkets with many outlets.
€ Highway Fishmongers Morning Period (6:00 am to 12:00 noon).
Ω Highway Fishmongers Evening Period (12:01 pm to 6:00 pm).
Љ Fresh Fish muscle tissue {bought directly from the fishing boats (Cocorite Fishing Port, Trinidad, W.I.)} and stored at -15°C; 1°C; and 29°C
Table 3: Total volatile acids {ppm} determined in the muscle tissue of Scomberomorus brasiliensis (Carite) purchased from four (4) locations and stored under different conditions.
The fish processing industry has an ongoing quest to seek a reliable method for assessing freshness measurements of the fresh starting material and ensuring that the fish products will not deteriorate when distributed and sold in retail markets or at the fish stales. An ideal method should be fast and accurate, inexpensive, nondestructive, and applicable to all fish samples; it should associate freshness quality as a factor of time and storage temperatures immediately after the harvest and should, therefore, provide the platform for estimating shelf-life.
The present study investigated the TVA and TVB assays as suitable spoilage indicators when compared with the other commonly used indicator such as hypoxanthine concentrations. Advantages of using a flow injection Analysis – Gas Diffusion (FIA-GD) method is relatively rapid (throughput=6 samples/hr), reproducible, sensitive, economical and very efficient separation of the analyte from sample components that may otherwise interfere with the detection method. All the fish samples in this study purchased at the four different locations Supermarket (Single and chain operators), Highway fish mongers (AM & PM), and the samples stored -15°C and 1°C were classified as edible [TVA and TVB] <220 ppm. All samples stored at 29°C were found to be inedible >220 ppm, based on their TVA and TVB concentrations found the fish muscle tissues of Scomberomorus brasiliensis (Carite) purchased and stored under different conditions and temperatures over a twelve month period.
Citation: Balladin DA (2020) Indicators of Fish Freshness: Total Volatile Acids and Bases. J Oceanogr Mar Res 8:199. doi: 10.35248/2572-3103.20.8.199
Received: 11-May-2020 Accepted: 27-May-2020 Published: 03-Jun-2020 , DOI: 10.35248/2572-3103.20.8.199
Copyright: © 2020 Balladin DA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.