Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2025)Volume 16, Issue 1
Objective: This study evaluated the use of cross-linked HA (EVOFill) for muscle augmentation.
Methods: The study was conducted with 48 patients of both sexes, aged between 22 and 40 years, without overweight, comorbidities, smoking habits, or oral medication use. Two types of hyaluronic acid (EVOFill Contour) were used: one for intramuscular filling and the other for subcutaneous tissue. The applications were performed in the gluteal muscles, quadriceps femoris, biceps femoris, calves, biceps, triceps, trapezius, and pectoral muscles using a hybrid technique, partially in the muscle and partially in the subcutaneous tissue. Applications were performed every two years. Additional treatments were performed every two months, if necessary, to correct asymmetries. Reapplications were conducted between 18 and 24 months.
Results: The results demonstrated a significant increase in muscle volume and a high satisfaction rate among patients and physicians after two years of treatment. Patients reported 100% satisfaction, and measurements confirmed the volume increase in the treated areas. Similar studies utilizing HA for gluteal augmentation also yielded satisfactory results, with participants reporting 100% satisfaction six months post-treatment, without adverse effects. HA fillers have proven to be highly effective, as evidenced by the data, owing to their excellent viscoelasticity, high water retention capacity, biocompatibility, and hygroscopic properties. Even though the use of HA for gluteal augmentation is considered safe, studies indicate that the safest site for injecting the compound is the subcutaneous fat. This is because, in this region, there are no vessels with a diameter greater than 2 mm or significant structures that could be susceptible to complications. In contrast, the intramuscular and sub muscular regions of the glutes contain nerves and blood vessels with larger calibers, rendering procedures in these areas riskier.
Conclusion: Despite the lack of results in the literature, our study confirms the efficacy and safety of cross-linked HA for muscle volume enhancement without adverse effects, highlighting the need for further research to expand the evidence base.
Muscle volume; Fillers; Hyaluronic acid; Aesthetics; Hyaluronic acid; Dermatological procedures; Crosslinked HA
Several types of dermal fillers are available today including polyacrylamide gel, polymethylmethacrylate, and Hyaluronic Acid (HA). In the late 1990s, HA-based fillers were introduced to the market as facial aesthetic injectable using minimally invasive techniques. Moreover, HA has been employed as a therapeutic tool to enhance the repair of damaged tissues [1,2].
HA belongs to the Glycosaminoglycan (GAG) family, which consists of heteropolysaccharides formed by repeating units of D-glucuronic acid and N-acetyl-D-glucosamine, linked via β (1→4) glycosidic bonds. The structure of HA differs from other GAGs due to its longer chain and absence of sulfate groups. Studies have shown that high-molecular-weight HA (>1000 kDa) plays a significant role in tissue regeneration and morphogenesis under physiological conditions. Additionally, it exhibits anti-inflammatory and antiangiogenic properties [3]. This is attributed to HA's involvement in signalling processes, interacting with multiple membrane receptors including TRPV1 and CD44, which are associated with inflammatory processes and nociception [4-8].
There are various commercially available HA that differ in their origin (natural or synthetic), crosslinking (linear or cross-linked), chain length (low or high molecular weight), sterilization process (heat or ultrafiltration), dosage, and the presence or absence of associated adjuvants. Cross-linked HA are three-dimensional structures of interconnected HA chains, typically administered as a single dose in most cases. These formulations provide slower degradation and extended joint residence time [9,10].
This carbohydrate is a key component of the extracellular matrix and is produced by most cells in the body. Approximately 27% of HA is found in the skin and connective tissue, while about 8% is located in muscles [11,12]. In muscle tissue, HA is present in the endomysium, perimysium, and epimysium, where it plays an essential role in lubrication and lateral force transmission during muscle contraction [12,13]. Moreover, high molecular weight HA can neutralize free radicals and reduce inflammation and pain [3,8,14].
In vitro studies indicate that HA may play a significant role in muscle repair by stimulating myoblast migration [15]. Additional findings demonstrate that HA levels dynamically change in response to hypertrophic stimuli, with various cells contributing to this adaptive mechanism in skeletal muscle. These data support the notion that HA plays a crucial role in myogenesis [8,15].
A study conducted in mice revealed that systemic administration of hyaluronidase can disrupt skeletal muscle architecture, inducing sensitivity in primary muscle afferents and promoting pain like behaviours. This study highlighted the importance of HA for the proper functioning of muscle tissue [8].
Hyaluronic acid has been successfully used for gluteal augmentation, yielding satisfactory outcomes [16]. A study involving sixty patients (55 women and 5 men) with a mean age of 35.5 years who underwent gluteal augmentation with HA, demonstrated a notably high rate of aesthetic improvement ("very good" and "good") as assessed by both patients and doctors. This improvement was observed within 7 to 14 days (94%) and remained consistent at 3 to 6 months (100%). The injection was performed just above the fascia of the gluteus maximus muscle. Thus, intramuscular injection has shown promise for this application [16].
Other studies have demonstrated that the delivery of satellite cells within a photoreticulated hyaluronic acid-based hydrogel into the partially ablated tibialis anterior of C57BL/6J mice significantly improved muscle structure and the number of new myofibers [17].
In this context, our study aims to report a series of cases involving muscle augmentation using hyaluronic acid.
A study was conducted with 48 patients of both sexes, aged between 22 and 40 years, without overweight, comorbidities, smoking habits, or oral medication use. HA application was performed using a hybrid technique, in which part of the product was injected into the muscle and part into the subcutaneous tissue. Two types of hyaluronic acid (EVOFill Contour) were used: one for intramuscular filling and the other for subcutaneous tissue. Both products shared the same formulation, with a concentration of 20 mg/mL and the same crosslinking agent.
The procedure was carried out in four phases:
Stage I: Preoperative markings
Preoperative markings were made to identify the treatment areas. The areas were determined based on aesthetic and functional assessments specific to each patient. The anatomy of each region was carefully considered, particularly the vascular and neural structures to avoid arterial occlusion or compartment syndrome. Application was also avoided over nerve trunks.
The markings followed the divisions:
Female gluteus-intramuscular application: The region was divided into two areas by a vertical line, positioned 6 cm from the intergluteal fold. The areas were then further subdivided by a lower horizontal line at the pelvic floor and a second line equidistant from the previously marked lower horizontal line and the highest point of the gluteus. A halo of approximately 6 to 8 cm was marked with a red pen, indicating the application site. The central point was the intersection of the vertical line and the posterior horizontal line (Figure 1A).
Male gluteus-intramuscular application: The region was divided into two areas by a vertical line, positioned 7 cm from the intergluteal fold. The areas were then further subdivided by a lower horizontal line at the pelvic floor and a second line equidistant from the previously marked lower horizontal line and the highest point of the gluteus. A halo of approximately 6 to 8 cm was marked with a red pen, indicating the application site. The central point was the intersection of the vertical line and the posterior horizontal line (Figure 1B).
Female gluteus-subcutaneous application: The same central point mentioned above is used, from which lines were drawn at a 15-degree angle between them, forming a 180-degree grid, resulting in 12 lines at which the subcutaneous injections were made. Next, the sacral triangle was marked in blue, and the shape of the gluteus, below the lower horizontal line (previously marked) was outlined in green, indicating the area at which the filler was applied (Figure 1C).
Male gluteus-subcutaneous application: The region was divided into two areas by a vertical line, positioned 7 cm from the intergluteal fold. A horizontal line was drawn 3 cm above the median line of the gluteus. A halo of approximately 6 to 8 cm was marked with a red pen, indicating the application site. The central point was the intersection of the vertical line and the posterior horizontal line (Figure 1D).
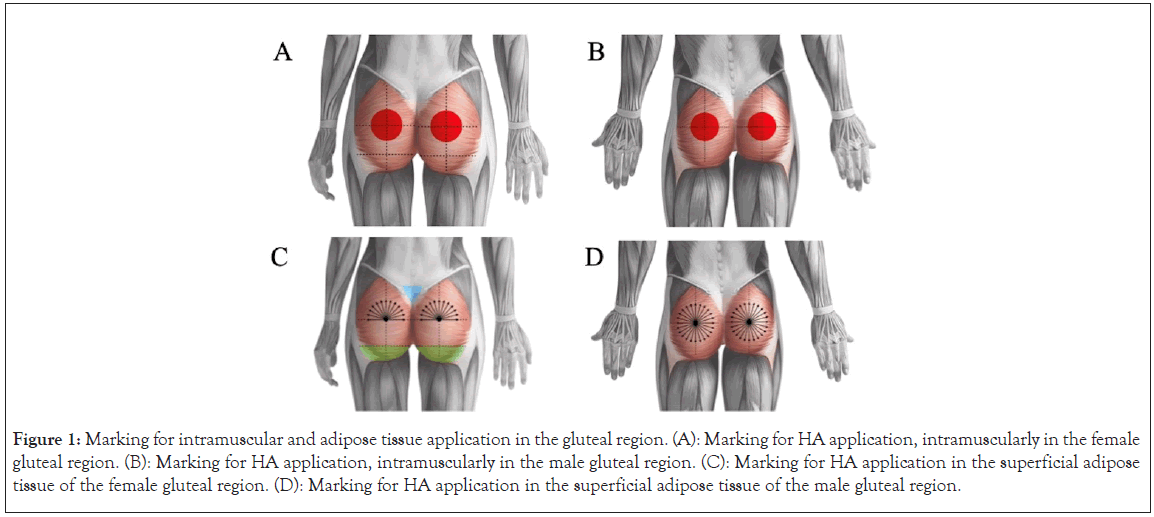
Figure 1: Marking for intramuscular and adipose tissue application in the gluteal region. (A): Marking for HA application, intramuscularly in the female gluteal region. (B): Marking for HA application, intramuscularly in the male gluteal region. (C): Marking for HA application in the superficial adipose tissue of the female gluteal region. (D): Marking for HA application in the superficial adipose tissue of the male gluteal region.
Biceps: The biceps brachii has two proximal heads with different insertions on the scapula and one distal insertion. For the application was intramuscular, the most distal point of the biceps was marked in red by drawing an ellipse, with the major axis measuring 5 cm and the minor axis measuring 2.5 cm (Figure 2A).
Triceps: The triceps brachii is a large muscle with three heads: the long head, the medial head, and the lateral head. For intramuscular application, the marking was made in red, focusing on the medial and lateral heads, by drawing an ellipse with a major axis of 5 cm and a minor axis of 2.5 cm (Figure 2B).
Shoulder-intramuscular application: In the shoulder was performed in the deltoid muscle (Figure 2C). This muscle was divided into three parts due to the different insertion and attachment points of the muscle fibers: the clavicular (from which the anterior muscle fibers arise), acromial (origin of the external muscle fibers), and scapular (attachment of the posterior muscle fibers). For the application, the clavicular region, which was the most anterior, is marked first, followed by the acromial (middle) and the scapular (posterior) region. The marking was done in red, drawing ellipses of 4 cm x 2 cm in each of these portions (Figure 2C).
Pectoral-intramuscular application: It was performed 2 cm above the nipple and 2 cm medially, with a central point marked. From this point, 12 lines were drawn, each at a 15-degree angle to the others, creating a 180-degree area with a length of 4–5 cm (Figure 2D).
Pectoral-subcutaneous application: The central point was the same as described above. Lines were drawn radiating from this point at a 15-degree angle, creating a 360-degree area (Figure 2E).
Figure 2: Marking for application in the biceps, triceps, shoulder, and male pectoral region. Marking for HA application, intramuscularly in the (A): Biceps; (B): Triceps; (C): Shoulder; (D): Pectoral region and (E): Marking for HA application in the superficial adipose tissue of the pectoral region.
Trapezius-intramuscular application: It was performed in the descending part of the trapezius muscle, by drawing an ellipse with dimensions of 4 cm x 2 cm (Figure 3).
Figure 3: Marking for application in the trapezius muscle. Marking for HA application in the descending region of the trapezius.
Posterior thigh: The application in this region was exclusively subcutaneous, with no intramuscular injection. The medial region of the muscle is marked, and the central point was made 6 cm below. From this point, 4 orange marks were made in the distal direction (Figure 4A and Figure 4B).
Thigh anterior surface: The application was performed in the muscular plane, specifically on the vastus lateralis and vastus medialis, followed by subcutaneous injection over both the vastus medialis and lateralis, as well as the vastus intermedius (Figure 4C–Figure 4F). In female patients, the application was performed on the inner part of the thigh in the subcutaneous layer (Figure 4C and Figure 4D).
Figure 4: Marking for application in the anterior and posterior thigh regions, (A and B): Marking for HA application in the superficial adipose tissue of the posterior thigh in females (A) and males (B); (C): Marking for HA application, intramuscularly in the anterior thigh of a female; (D) Marking for HA application in the superficial adipose tissue of the anterior thigh in a female; (E): Marking for HA application, intramuscularly in the anterior thigh of a male and (F): Marking for HA application in the superficial adipose tissue of the anterior thigh in a male.
Calf muscle-intramuscular application: First, the lowest point of the muscle was marked, both for the medial and lateral fascicles, by drawing an ellipse measuring 6 cm x 3 cm (Figure 5).
Figure 5: Marking for application in the calf, (A): Marking for HA application, intramuscularly in the female calf and (B): Marking for HA application, intramuscularly in the male calf.
Stage II: Asepsis and anesthetic infiltration
Asepsis of the application sites was performed with an antiseptic solution of alcoholic chlorhexidine (0.5% chlorhexidine digluconate), followed by an anesthetic button with 2% lidocaine. Next, local anesthesia was applied at the filling site using Klein's Solution (100 ml of saline solution, 10 ml of sodium bicarbonate, 1 ml of epinephrine, and 20 ml of lidocaine). To prevent bleeding after vasoconstriction, 18G-70 mm and 16G-70 mm cannulas (EVO CANNULA SCULPT; MODELS: MKW1670 and MKW1870) from Evopharma were used, depending on the patient and the application site. The cannulas are imported and distributed by Evopharma Ltda.
Stage III: HA Injection
For the procedure, cross-linked hyaluronic acid (EVOFill) was used in volumes ranging from 20 to 150 mL. Applications were performed every two years, using Evopharma cannulas of 18G-70 mm and 16G-70 mm (MODELS: MKW1670 and MKW1870), depending on the patient and the application site. The cannulas are imported and distributed by Evopharma Ltda. The application areas included the glutes, quadriceps femoris, biceps femoris, calves, biceps, triceps, trapezius, and pectoral muscles. Additional treatments were performed every two months, if necessary, to correct asymmetries. Reapplications were conducted between 18 and 24 months.
Stage IV: Evaluation
A patient satisfaction assessment was conducted using a scale of 1 to 5, based on the Customer Satisfaction Score (CSAT) metric, where 1 represents "very dissatisfied" and 5 represents "very satisfied." Additionally, pain was evaluated on a scale from 1 to 10. Measurements of the applied areas were carried out every 2 months.
After the treatment period, it was possible to observe a volumization of the applied areas, with a considerable increase in muscle tone in all areas of application (Figures 6–8). The assessed patients reported a satisfaction level of 5 in 100% of the cases, after 2 years of treatment. The level of satisfaction from the doctors was also 5 in 100% of the cases. Measurements from the patients showed a significant increase in the filled areas (Figures 7 and 8). Moreover, no adverse effects were observed or reported by the patients.
Figure 6: Photodocumentation of two representative cases of hyaluronic acid application in the anterior thigh region of a female patient, (A): Region before application; (B): Region two months after application, showing a significant increase in muscle volume and (C): Photodocumentation after application in the anterior region of the left thigh.
Figure 7: Photodocumentation of two representative cases of hyaluronic acid application in the female gluteal region, (A): Region before application; (B): Region two months after application, showing a significant increase in muscle volume; (C): Region before application and (D): Region two months after application, showing a significant increase in muscle volume.
Figure 8: Representative photodocumentation of hyaluronic acid application in the male biceps region, (A): Region before application and (B): Region two months after application, showing a significant increase in muscle volume.
The strength of skeletal muscle is not solely transmitted along the muscle fibers but also through the Extracellular Matrix (ECM) [18]. Recent studies have sought to accurately describe the structure of the muscle tissue ECM, aiming to characterize its passive mechanical properties [18]. The muscle ECM is composed mainly of type I and type III collagen [18][19][20]. Furthermore, it contains proteoglycans, Glycosaminoglycans (GAGs), and glycoproteins. Hyaluronic Acid (HA) is an abundant GAG in the muscle ECM, particularly in the endomysium, perimysium, and epimysium. In these regions, HA plays an important role in lubrication and lateral force transmission during muscle contraction [12][13][18].
The results obtained from this study showed that the application of cross-linked HA (EVOFill) was able to increase muscle volume without presenting adverse effects. The observed effect occurs because the product exhibits excellent viscoelasticity, high water retention capacity, biocompatibility, and hygroscopic properties [21]. Studies have shown that even at low concentrations (0.1%), HA chains can provide high viscosity, lubrication, cushioning, and stabilization of the joint structure [21][22][23][24]. Similar studies have shown that hyaluronic acid was effective in increasing gluteal volume in patients, with high safety and precision. These studies included 60 participants, with 55 (91.6%) women and 5 (8.4%) men. The volume of HA injected ranged from 15 to 100 ml. Aesthetic improvement ratings were very good or good in 94% of the cases. After 3 to 6 months following treatment, 100% of patients reported improvement in their perception of the results [16]. In our study, 100% of the female patients reported aesthetic improvement, with no adverse effects reported.
The increase in gluteal volume was also observed in 43 patients who received hyaluronic acid in this region. The results show that the treated patients reported significant satisfaction with the treatment. Better outcomes were observed in patients who received a larger volume of the product. Additionally, no serious complications were observed during or after the treatment [25].
Even though the use of HA for gluteal augmentation is considered safe, studies indicate that the safest site for injecting the compound is the subcutaneous fat. This is because, in this region, there are no vessels with a diameter greater than 2 mm or significant structures that could be susceptible to complications. In contrast, the intramuscular and submuscular regions of the glutes contain nerves and blood vessels with larger calibers, rendering procedures in these areas riskier [26].
Despite the lack of results in the literature, our study demonstrated that the procedure for increasing muscle volume with HA can be extremely effective and satisfactory for patients. In this context, further studies should be conducted to describe the effects in a larger patient population.
The application of cross-linked hyaluronic acid (EVOFill) was effective in increasing muscle volume in the evaluated patients over a two-year period. During this time, all patients reported satisfaction with the treatment. Furthermore, the applications in the aforementioned regions proved to be safe and did not induce any side effects in the participants.
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Salomao Junior A, Alfenas TD, Braz AV, Mourao CN, Corbett J, Luna ACL, et al. (2025). Increasing muscular volume with hyaluronic acid. J Clin Exp Dermatol Res. 16:685
Received: 17-Mar-2025, Manuscript No. JCEDR-25-37056; Editor assigned: 17-Mar-2025, Pre QC No. JCEDR-25-37056 (PQ); Reviewed: 25-Apr-2025, QC No. JCEDR-25-37056; Published: 04-Apr-2025 , DOI: 10.35841/2155-9554.25.16.685
Copyright: © 2025 Salomao Junior A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.