Forest Research: Open Access
Open Access
ISSN: 2168-9776
ISSN: 2168-9776
Research Article - (2018) Volume 7, Issue 1
Field experiment was carried out at the Experimental Farm of the Salinity and Alkalinity laboratory, Sabaheia- Alexandria, to quantify growth, biomass and chemical composition on responses of lebbeck and chinaberry to three levels of drought stress and three organic amendments after two successive seasons of 2015 and 2016. The results indicated that well-watered treatment had the higher values of height growth, stem diameter, leaves number, shoots dry weight, shoots: roots (S: R) ratio, total chlorophyll and total carotenoides. On the other hand, severe treatment (50% field capacity) resulted in the highest values of roots dry weight, proline and total phenols contents for both tree species. Compost was better to enhance the growth parameters of lebbeck while, humate was the better to increase growth parameters of chinaberry. Total chlorophyll and total carotenoides of the two species were enhanced by adding humate. The interaction results indicated that the highest values of growth parameters of lebbeck resulted from compost combined with moderate or well-watered treatments. While humic acid combined with well-watered or moderate treatments were the best for growth of chinaberry seedlings.
<Keywords: Drought resistant; Organic amendment; Lebbeck; Chinaberry
Lebbeck (Albizia lebbeck L. Benth.) belongs to family Fabaceae. It is a nitrogen-fixing tree, a valued species that have a good quality hardwood timber, fuel-wood and charcoal, and honey (source of nectar and pollen). The extensive, shallow root system makes it a good soil binder and suited to soil conservation and erosion control. It grows on less fertile soils, very drought tolerant and being found in areas with rainfall as low as 300-400 mm yr-1 [1]. Chinaberry (Melia azedarach) belongs to family Meliaceae, and is highly adaptable and tolerates a wide range of climatic and soil conditions and is generally found in tropical, sub-tropical and warm-temperate climates mostly associated with seasonally dry conditions [2]. Chinaberry has a shallow root system and rapid growth species. The timber is soft, strong, easily worked and light in weight [3,4]. Wood has been used for framing and boards, flooring, cabinet work, fixtures and interior joinery [5].
To prevent water, stress a plant can regulate transpiration by closing the stomata. As stomata are closed, photosynthesis will become hindered, and water transport is also disturbed, leading to turgor loss in phloem and weakened phloem loading [6]. The weakened phloem loading prevents carbohydrates from being transported from sources to sinks and down regulated photosynthesis prevents the production of new carbohydrates, causing carbon starvation. Drought-affected trees are prone to reduced growth [7] as well as increased allocation of matter to roots [8].
Composts have favorable effects on plant growth through improving chemical and physicochemical soil properties, improving water deficiency and providing soil with essential macro and micronutrients [9]. Meanwhile, composted materials need to be well characterized for nutrient values, stability, and other properties for the support of tree growth. Humic acid has anti-stress effects under abiotic stress conditions such as drought [10]. It can reduce free radicals resulting from drought which damage lipids, proteins and DNA within plants cells [11]. Humic acid is highly beneficial to both plant and soil; it is important for increasing microbial activity, it is considered as a plant growth bio-stimulant, an effective soil enhancer; it promotes nutrient uptake as chelating agent and improves vegetative characteristics, nutritional status and leaf pigments [12,13]. On the other hand, Tan [14] mentioned that humic substances have indirect effects involve improvements of soil properties such as aggregation, aeration, permeability, water holding capacity, micronutrient transport and availability. The mechanism of Humic action remains unclear. The main reason seems to be its stochastic nature. In contrast to common biological macromolecules, which are synthesized by a living organism according to the information encoded in DNA (nuclear acids, proteins, enzymes, antibodies etc.), Humic are the products of stochastic synthesis. They are characterized as polydisperse materials having elemental compositions that are nonstoichiometric, and structures, which are irregular and heterogeneous [15]. The objective of this study was investigated the tolerance of lebbeck and chinaberry transplants to drought by adding some organic amendments under sandy loam soil grown in Alexandria after two successive seasons.
The present study was conducted in concrete basins with dimensions 0.5 × 0.5 × 1.0 m at the Experimental Farm of Soil Saline and Alkaline Research Laboratory (Salinity Lab) at Sabaheia, Alexandria during 2015 and 2016 seasons. Two species of trees transplants (Albizia lebbeck and Melia azedarach) were examined separately for this study. Each tree experiment included 9 treatments with three replicates in a split plot design, which were the combination of 3 drought stress levels, as sub-plot, interacted with 3 types of organic substances as the main plot. After tilling and weed removing from the sandy loam soil, the following three types of organic substances thoroughly incorporated with the soil of concrete basins to a depth of 0.6 m: (1) control, (nonamended), (2) compost, (prepared in the backyard of the Soil Saline and Alkaline Research Laboratory, (Table 1) which added by the rate of 20 m3 fed-1 (1 kg basin-1) and (3) humic acid (commercial powder humate contain 86% humic acids as a potassium humate and 5% K2O w/w, applied by the rate of 5 kg fed-1). Then the tree transplants (oneyear- old) were planted in first week of March 2015. During the first month, all transplants were kept well-watered. Thereafter, in April 2015 these watering regimes were imposed for 87 weeks: (1) well-watered, (100% of field capacity), (2) moderate stress, (75% of field capacity) and (3) severe stress (50% of field capacity). The study was ended in the last week of October 2016 for two growing seasons.
| Criterion | Value | Criterion | Value |
|---|---|---|---|
| Bulk density, Mg m-3 | 0.54 | Total K*, % | 1.12 |
| Moisture content at 65°C, % | 29.41 | Total P*, % | 0.66 |
| Moisture content at 105°C, % | 38.16 | Total N*, % | 1.14 |
| Dry matter, % | 61.84 | ||
| pH, 1:10*(H2O) | 7.11 | Available K*, % | 0.45 |
| EC, dsm-1 1:10*(H2O) | 3.25 | Available P*, % | 0.18 |
| Soluble cations, meq L-1 | NH4-N+NO3-N*, mg/kg | 108.66 | |
| Ca++ | 7.32 | ||
| Mg++ | 2.66 | Organic carbon (O.C) *, % | 28.66 |
| Na+ | 11.91 | ||
| K+ | 9.80 | C/N | 25.14 |
| SAR | 5.33 | C/P | 43.42 |
| Soluble anions, meq L-1 | C/K | 25.59 | |
| HCO3- | 1.32 | ||
| Cl- | 11.89 | ||
| SO4- - | 18.48 |
*=Dry matter basis
Table 1: Main characteristics of dry compost used in the study.
The direct gravimetric technique was used to quantify soil moisture content of the experimental soil (IAEA, 2008). This technique was repeated every week interval to adjust the requiring water until the end of the experiment to confirms that stress levels were well maintained. Soil samples were taken from concrete basins at 0-30 and 30-60 cm depth and mixed to form composite soil samples with three replicates at the beginning of the growing season before application of the organic substances. The soil samples were air-dried, crushed and sieved through a 2 mm sieve. The portion less than 2 mm (fine earth separates), were used to carry out the laboratory analysis.
Soil pH and electrical conductivity were determined, soil texture was detected using the Hydrometer method [16]. The bulk density of the soil samples was determined using core method as described by Blake and Hartge [17]. Soluble carbonates and bicarbonates were determined volumetrically according to Jackson [18]. Soluble chlorides were determined by according to Richards [19]. Also, the sodium adsorption ratio (SAR) value in the soil calculated by the following formula:
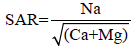
Available nitrogen in soil was determined [20], total available was determined by the Micro-Kjeldahl technique [21], available phosphorous and potassium were determined [22]. Also, cation exchange capacity (CEC) was determined by the neutral (pH 7.0) NH4OAc saturation method [23]. Total organic carbon (TOC) was measured in the field moist soil samples using wet oxidation procedure with adding potassium chromate [24]. Organic matter content (OM) in the soil samples was calculated according to the following relationship: OM%=TOC% × 1.724, Where: 1.724=Conversion Factor [25].
The samples were analyzed for soil texture, bulk density, pH, EC, organic matter content, available nitrogen, phosphorous and potassium, cation exchange capacity (CEC) and soil labile carbon (LC) based on KMnO4 oxidation [26] was measured as well. The physical and chemical analyses of the experimental soil are presented in Table 2.
| Criterion | Value | Criterion | Value |
|---|---|---|---|
| Texture classes | Sandy Loam | Soluble anions, m mol/L | |
| Sand, % | 79.85 | HCO3- | 0.56 |
| Silt, % | 8.64 | Cl- | 1.57 |
| Clay, % | 11.51 | SO4-- | 0.42 |
| Bulk density, Mg m-3 | 1.65 | SAR | 1.54 |
| Field capacity (F.C.), % | 10.00 | ||
| Saturation water content (S.P), % | 25.8 | CEC, c mol Kg-1 | 5.70 |
| Moisture content, Өw, % | 1.31 | Organic carbon (O.C), g Kg-1 | 0.09 |
| Total CaCO3, % | 0.20 | NH4-N+NO3-N, mg Kg-1 | 9.08 |
| pHw, 1:2.5 (water suspension) | 7.73 | Ave. P, mg Kg-1 | 5.11 |
| EC, 1:2.5 (water extract) dSm-1 | 0.17 | Ave. K, mgKg-1 | 8.31 |
| Soluble cations, m mol/L | Labile carbon (LC), mg Kg-1 | 8.71 | |
| Ca++ | 0.83 | ||
| Mg++ | 0.31 | ||
| Na+ | 1.16 | ||
| K+ | 0.25 |
Table 2: Main characteristics of initial soil sample for the concrete basins soils.
The fresh leaves collected in mid-August each season to estimate total chlorophyll content according to Arnon [27] and carotenoids by following the method of Robbelen [28]. In the last week of October, the leaves number was counted. Also, the transplants were cut and sorted to leaves, stem and roots. The root systems were cut and cleaned from soil by rinsing with tap water thereafter, fresh and dry biomasses of shoots (leaves+stem) and roots of each replicate were measured then, shoot: root ratio was calculated. The leaves of each replicate were washed with distilled water, oven dried at 65°C for 72 h and then ground in a stainless-steel mill and the powder stored for elemental analysis. Free proline was determined according to Bates et al. [29] as well as, total phenolic compounds was determined spectrophotometric by using the Folin-Denis method according to Cheng and Hanning [30] as mg of gallic acid per gram of leaves dry weight.
Relative growth rate (RGR), which expresses growth as a function of height per unit interval of time, indicate the degree to which water stress affected height increment, was calculated for plants of each treatment as:
RGR=(log H2-log H1)/T,
Where: H2=Final plant height; H1=Initial plant height; T=number of weeks.
All data were subjected to split plot analysis of variance using CoStat statistical package for Windows. Differences between means of investigated parameters were tested with Duncan’s multiple range test (P ≤ 0.05).
Growth
Table 3 shows that the severe drought stress treatment (50%) decreased the height growth, stem diameter and height relative growth rate (RGR) of Albizia lebbeck transplants by 43.1, 25.0 and 56.74% less than well-watered treatment, respectively. The transplants also, reduced their leaves number when stressed under severe drought by 34.0% less than well-watered treatment (Table 3). Although the Moderate stress treatment (75% of field capacity) was slightly reduced the leaves number, but it significantly was similar with the well-watered treatment. On the other hand, the compost was more effectively as minimized the effect of drought stress on height growth and height (RGR). While, the stem diameter significantly not affected by the organic amendments. Generally, compost elongated the height growth by 1.3 and 1.5-fold, respectively than humic that significantly did not differ than non-amended treatment. Instead, both humic and compost minimized the effects of drought stress on leaves growth therefore, compost increased the leaves number by 16.2% more than non-amended transplants. The data of interaction between drought stress and organic substances revealed that compost and humic had the highest values under each stress levels.
| Treatment | Cont. | Compost | Humic | Mean | Cont. | Compost | Humic | Mean | |
|---|---|---|---|---|---|---|---|---|---|
| Height growth (cm) | Stem diameter (cm) | ||||||||
| Well watered | 225.00bc | 275.00a | 228.00b | 242.61a | 4.35ab | 4.68a | 4.29ab | 4.44a | |
| Moderate | 195.00c | 230.00b | 210.00bc | 211.67b | 4.72a | 4.73a | 4.51a | 4.65a | |
| Severe | 114.00d | 195.00c | 105.00d | 138.00c | 3.24c | 3.48bc | 3.27c | 3.33b | |
| Mean | 178.00b | 233.33a | 180.94b | 4.10a | 4.30a | 4.02a | |||
| Leaves No. | RGR cm week-1 | ||||||||
| Well watered | 65.67ab | 73.33a | 67.67ab | 68.89a | 1.57b | 2.15a | 1.61b | 1.78a | |
| Moderate | 54.33c | 67.33ab | 64.00b | 61.94a | 1.04de | 1.44bc | 1.21cd | 1.23b | |
| Severe | 43.00d | 48.67cd | 44.67d | 45.44b | 0.69ef | 1.02de | 0.59f | 0.77c | |
| Mean | 54.33b | 63.11a | 58.78ab | 1.10b | 1.53a | 1.13b | |||
Means followed by a similar letter within a column or row are not significantly different at the probability level 0.05 using Duncan’s Multiple Range Test.
Table 3: Influence of drought levels, organic amendments and their interaction on growth of Albizia lebbeck transplants after two seasons.
Moderate and severe drought stress slightly, decreased the height growth of Melia azedarach transplants by 7.6 and 19.6% than wellwatered treatment, respectively. However severe stress impressively thinned the stem diameter more than moderate and well-watered transplants (Table 4). Data illustrated that severe drought decreased the leaves number of M. azedarach by 48.0% less than well-watered transplants. Also, severe drought achieved the less RGR by 0.76 cm week-1. Humic markedly enhanced the height growth and stem diameter by 13.0 and 66.3% than non-amended treatment, respectively whereas compost had non-significant effect on height growth. As well as, humic had the priority to minimize the injuries of drought stress on leaves growth of M. azedarach as increased leaves number by 46.2% more than non-amended transplants while compost had non-significant effects on leaves growth. Likewise, humic achieved the highest RGR by 1.13 cm week-1 for M. azedarach transplants. The interaction effect between drought stress levels and organic substances was obvious on all growth parameters of M. azedarach particularly humic that was superior to compost under all drought levels.
| Treatment | Cont. | Compost | Humic | Mean | Cont. | Compost | Humic | Mean | |
|---|---|---|---|---|---|---|---|---|---|
| Height growth (cm) | Stem diameter (cm) | ||||||||
| Well watered | 188.00ab | 190.00ab | 196.00a | 191.33a | 2.33de | 2.66cd | 4.16a | 3.05a | |
| Moderate | 165.00bc | 171.67abc | 194.00a | 176.89b | 2.49cd | 2.82c | 3.26b | 2.86a | |
| Severe | 141.00d | 152.33cd | 168.00bc | 153.78c | 1.16f | 2.13e | 2.52cd | 1.94b | |
| Mean | 164.67b | 171.33b | 186.00a | 1.99c | 2.53b | 3.31a | |||
| Leaves No. | RGR cm week-1 | ||||||||
| Well-watered | 29.67c | 35.33b | 48.33a | 37.78a | 1.15ab | 1.17ab | 1.24a | 1.19a | |
| Moderate | 23.33d | 23.33d | 31.00bc | 25.89b | 0.89bcd | 0.96abc | 1.22a | 1.02b | |
| Severe | 17.00e | 18.67de | 23.00d | 19.56c | 0.61d | 0.74cd | 0.92bc | 0.76c | |
| Mean | 23.33b | 25.78b | 34.11a | 0.88b | 0.96b | 1.13a | |||
Means followed by a similar letter within a column or row are not significantly different at the probability level 0.05 using Duncan’s Multiple Range Test.
Table 4: Influence of drought levels, organic amendments and their interaction on growth of Melia azedarach transplants after two seasons.
The results of drought stress matched with Jing et al. [31] on poplar transplants that the height growth and basal diameter decreased with increasing drought levels. With the increasing extent and duration of drought stress, the different parameters of the leaves decreased gradually under light drought stress, while decreased rapidly under both moderate and severe drought stress. Also, they explained that under moderate and severe drought stress, the photosynthetic efficiency decreased significantly, and the oxidative enzyme defense system was damaged remarkably. Improving the growth of Albizia lebbeck and Melia azedarach with applying humic confirmed by those obtained by Vaughan et al. [32], who showed evidence of stimulation on plant growth by humic substances and consequently increased yield by acting on mechanisms involved in: cell respiration, photosynthesis, protein synthesis, water, and nutrient uptake, enzyme activities. Also, this effect may have described by Zhang and Ervin [33], who mentioned that humic acid contains cytokinins and their application resulted in increased endogenous cytokinin and auxin levels which possibly leading to improve growth.
Biomass
After two seasons, severe drought had substantially decreased the shoots dry biomasses of A. lebbeck by 53.95% less than well-watered transplants. Conversely, it extremely increased the dry weight of roots by 104.28% more than well-watered transplants (Table 5). The S: R ratio is often used to estimate relative biomass allocation between shoots and roots. With the increasing intensity of drought, shoots biomass decreased gradually whereas, roots biomass increased. These resulting in a decrease of S: R ratio so, the S: R in the severe drought was about two times higher than that in well-watered treatment. Although, both organic amendments boosted the shoots biomasses but humic was the higher to increase the dry shoots biomass of A. lebbeck by 12.9% more than non-amended transplants. On the other hand, the compost and humic had no significance effect on the dry roots biomass. Subsequently, humic and compost slightly improved S: R ratio for the transplants under drought stress with the same significant level.
| Treatment | Cont. | Compost | Humic | Mean | Cont. | Compost | Humic | Mean |
|---|---|---|---|---|---|---|---|---|
| Shoots DW. (g) | Root DW. (g) | |||||||
| Well-watered | 859.00bc | 1000.00a | 963.33ab | 940.78a | 201.63d | 183.54e | 175.72e | 253.63b |
| Moderate | 570.00d | 863.67bc | 751.34c | 728.33b | 353.86c | 357.75c | 349.80c | 353.80b |
| Severe | 436.67e | 471.34de | 391.67e | 433.22c | 619.96a | 474.23b | 460.12b | 518.11a |
| Mean | 621.89c | 778.33a | 702.11b | 391.82a | 405.17a | 328.55a | ||
| S:R ratio | ||||||||
| Well watered | 4.26b | 5.43a | 5.48a | 5.06a | ||||
| Moderate | 1.61d | 2.41c | 2.15c | 2.06b | ||||
| Severe | 0.71e | 0.99e | 0.85e | 0.85c | ||||
| Mean | 2.19b | 2.94a | 2.83a | |||||
Means followed by a similar letter within a column or row are not significantly different at the probability level 0.05 using Duncan’s Multiple Range Test.
Table 5: Influence of drought levels, organic amendments and their interaction on dry biomass of Albizia lebbeck transplants after two seasons.
The drought stress gradually decreased the shoots dry biomasses of M. azedarach from moderate to severe stress. Concurrently, the roots dry biomasses of moderate and severe treatments increased compared to the well-watered transplants without significant differences between them. These results leading to a low S: R ratio for severe and moderate drought by about 2.8 and 2.4 times less than the ratio of well-watered transplants, respectively (Table 6). On the other hand, humic was the best amendment to reduce the injury of drought on shoots biomass of M. azedarach followed by compost. Humic increased the dry biomasses of shoots and roots by 55.39 and 38.05% more than transplants in non-amended soil, respectively. Although humic slightly increased the S: R ratio, but there is not significant differences between the used amendments.
| Treatment | Cont. | Compost | Humic | Mean | Cont. | Compost | Humic | Mean | |
|---|---|---|---|---|---|---|---|---|---|
| Shoots DW. (g) | Root DW. (g) | ||||||||
| Well watered | 336.43bc | 384.33b | 490.33a | 403.70a | 70.00h | 67.40h | 101.54g | 79.65b | |
| Moderate | 236.83de | 320.50bcd | 380.17b | 312.50b | 118.76e | 153.70c | 169.63b | 147.36a | |
| Severe | 195.00e | 264.00cde | 323.33bcd | 260.78c | 110.55f | 184.48a | 142.02d | 145.68a | |
| Mean | 256.09c | 322.94b | 397.94a | 99.77c | 135.19b | 137.73a | |||
| S:R ratio | |||||||||
| Well watered | 4.81b | 5.71a | 4.83b | 5.12a | |||||
| Moderate | 1.99cd | 2.08cd | 2.24c | 2.11b | |||||
| Severe | 1.76cd | 1.43d | 2.28c | 1.82b | |||||
| Mean | 2.85a | 3.08a | 3.12a | ||||||
Means followed by a similar letter within a column or row are not significantly different at the probability level 0.05 using Duncan’s Multiple Range Test.
Table 6: Influence of drought levels, organic amendments and their interaction on dry biomass of Melia azedarach transplants after two seasons.
The results of biomasses are similar with Zwack and Graves [34] who frequently observed an increase in S: R ratio in maple cultivars under drought conditions. This decrease was mostly from a larger reduction in shoot growth than in roots by water deficit. In drought conditions, the growth inhibition outcome as a reduction in shoots biomass and increasing the roots biomass to enhance water uptake [35]. The mechanism of this re-distribution is believed to be associated with the accumulation of abscisic acid and a reduction in the cytokinin level [36] or maybe due to a greater osmotic adjustment in roots compared with shoots under drought conditions [37]. Also, Genhua et al. [38] stated that the high root-to-shoot ratio may help the survival of plants under drought conditions in the semiarid environment. Field soil moisture contents generally increase with soil depth; therefore, an extensive root system is able to access a larger soil volume to extract available water. The high results of humic on biomass agree with those obtained by Eissa et al. [12]; Ismail et al. [13] on pear transplants. Also, Chen et al. [39] suggested that the humic acid increased cell membrane, important for the transport and availability of micronutrients, nutrient uptake, stimulates viability, oxygen uptake, respiration, especially in roots, and photosynthesis. This may explain the increment in shoots biomasses. Among plant organs, root tissue is severely affected by drought due to its direct contact with drying soil. Among the most common responses to water stress are electron leakage through thylakoid membranes, damage of chlorophylls, decrease in antioxidant system, increase in production of H2O2, O2, lipid peroxidation and decrease in photosynthesis [40].
Chemical Composition
Table 7 reveal that drought stress slightly collapsed the total chlorophyll content of A. lebbeck leaves where moderate and severe droughts collapsed total chlorophyll by 3.1 and 14.0% less than wellwatered transplants, respectively. Although moderate drought and well-watered treatments significantly were similar, but severe drought only had substantially decreased the total carotenoids by 14.6% less than well-watered transplants. It is known that the trees use chemical strategy against stresses includes the synthesis and accumulation ofreveal that drought stress slightly collapsed the total chlorophyll content of A. lebbeck leaves where moderate and severe droughts collapsed total chlorophyll by 3.1 and 14.0% less than wellwatered transplants, respectively. Although moderate drought and well-watered treatments significantly were similar, but severe drought only had substantially decreased the total carotenoids by 14.6% less than well-watered transplants. It is known that the trees use chemical strategy against stresses includes the synthesis and accumulation of secondary metabolites, such as proline and phenolic compounds therefore, severe drought treatment slightly accumulated proline by 14.7% more than the well-watered. Also, the severe stress increased total phenolic compounds by 25.17% more than well-watered transplants. The humic and compost, minimized the injuries of drought stress on total chlorophyll and total carotenoids of A. lebbeck leaves (Table 7). The transplants treated with humic and compost had noticeable increase in total chlorophyll by 43.7 and 25.4% more than non-amended transplants, respectively. Likewise, both amendments enhanced the total carotenoids by 33.4 and 26.0% more than nonamended transplants, respectively. Instead, they significantly, by little, minimized the accumulation of proline by 17.2 and 14.2%, respectively less than non-amended transplants under drought stress. On the other hand, humic was the better in minimizing the accumulation of phenolic compounds by 16.56% less than non-amended transplants.
| Treatment | Cont. | Compost | Humic | Mean | Cont. | Compost | Humic | Mean | |
|---|---|---|---|---|---|---|---|---|---|
| Total chlorophyll (mg 100g-1 FW) | Total carotenoids (mg 100g-1 FW) | ||||||||
| Well watered | 292.74g | 313.54e | 396.65a | 334.31a | 31.65d | 35.95a | 36.56a | 34.72a | |
| Moderate | 261.14h | 342.28c | 368.57b | 324.00b | 30.31e | 32.99c | 36.20a | 33.17b | |
| Severe | 215.04i | 308.02f | 339.81d | 287.62c | 18.74f | 32.75c | 34.91b | 28.80c | |
| Mean | 256.31c | 321.28b | 368.34a | 26.90c | 33.90b | 35.89a | |||
| Proline (mg g-1 DW) | Phenols (mg g-1 DW) | ||||||||
| Well watered | 0.249b | 0.215efg | 0.209g | 0.224b | 61.45c | 50.33de | 49.85e | 53.88c | |
| Moderate | 0.224de | 0.221def | 0.212fg | 0.219b | 63.22c | 52.47d | 50.78de | 55.49b | |
| Severe | 0.310a | 0.234c | 0.227cd | 0.257a | 71.50a | 67.55b | 63.04c | 67.36a | |
| Mean | 0.261a | 0.224b | 0.216b | 65.39a | 56.78b | 54.56c | |||
Means followed by a similar letter within a column or row are not significantly different at the probability level 0.05 using Duncan’s Multiple Range Test.
Table 7: Influence of drought levels, organic amendments and their interaction on chemical compositions of Albizia lebbeck transplants after two seasons.
The chemical composition of M. azedarach transplants affected by drought as severe and moderate stresses collapsed the total chlorophyll by 24.8 and 13.3% less than well-watered transplants, respectively (Table 8). Also, the both levels of drought decreased total carotenoids by 19.5 and 10.2%, respectively. Severe drought stress extremely accumulated proline and total phenolic compounds in the leaves of M. azedarach by 66.2 and 16.45% followed by moderate stress that accumulated the proline by 32.4 and 3.88% more than well-watered transplants, respectively. Moreover, humic exceeded compost for minimizing the injuries of drought stress on leaves chemical compositions. Therefore, the total chlorophyll contents were higher than non-amended transplants by 36.7 and 30.16% for humic and compost, respectively. The both amendments were similar significantly in reduce the drought injury of total carotenoids, proline and total phenolic compounds accumulation in compare with non-amended transplants. The drought stress caused accumulation of proline and total phenolic compounds in A. lebbeck leaves which may indicate that these compounds play an important role in the adaptation of leaves to growth under stress conditions.
| Cont. | Compost | Humic | Mean | Cont. | Compost | Humic | Mean | ||
|---|---|---|---|---|---|---|---|---|---|
| Total chlorophyll (mg 100g-1 FW) | Total carotenoids (mg 100g-1 FW) | ||||||||
| Well watered | 227.68d | 288.93a | 298.78a | 271.80a | 24.72bc | 27.38a | 27.89a | 26.66a | |
| Moderate | 195.48e | 251.27bc | 260.39b | 235.71b | 21.61d | 24.70bc | 25.48b | 23.93b | |
| Severe | 159.11f | 217.67d | 236.80cd | 204.53c | 18.97e | 22.25d | 23.13cd | 21.45c | |
| Mean | 194.09c | 252.62b | 265.32a | 21.76b | 24.78a | 25.50a | |||
| Proline (mg g-1 DW) | Phenols (mg g-1 DW) | ||||||||
| Well watered | 0.157cd | 0.134d | 0.128d | 0.139c | 13.31de | 12.84ef | 12.51f | 12.89c | |
| Moderate | 0.221abc | 0.174bcd | 0.160cd | 0.184b | 15.24b | 12.68ef | 12.24f | 13.39b | |
| Severe | 0.267a | 0.183bcd | 0.238ab | 0.231a | 16.95a | 14.15c | 13.93cd | 15.01a | |
| Mean | 0.216a | 0.163b | 0.176b | 15.17a | 13.22b | 12.89b | |||
Means followed by a similar letter within a column or row are not significantly different at the probability level 0.05 using Duncan’s Multiple Range Test.
Table 8: Influence of drought levels, organic amendments and their interaction on chemical compositions of Melia azedarach transplants after two seasons.
The results of total chlorophyll and carotenoids are matched with Eissa et al. [12] and Ismail et al. [13] whom stated that humic improves leaf pigments. Also, the result of proline accumulation confirmed those obtained by Kulikova et al. [11] who found that humic can reduce free radicals resulting from drought which damage proteins within plants cells. Kramer and Boyer [41] revealed that plant cells have the ability, known as osmotic adjustment, to maintain turgor pressure. The adjustment implies accumulation of solutes in the cell, mostly compatible solutes, amino acids in the cytoplasm, and inorganic solutes in the vacuole. In many cases this is not just a decrease in water content as a passive response to the lower external water potential. Proline is one of the osmolytes, which increase faster than other amino acids in plants under water deficit stress and help the plants to maintain cell turgor [42].
Thus, proline accumulation can be used as a criterion for drought resistance assessment of varieties [43]. The increase of proline content in leaves of stressed plants may suggest a possible protective role of proline in improving photosynthetic activity by activating osmotic adjustment along the stress period, in order to maintain hydration status of the plants. This way led to increasing water uptake and retention to actively growing tissues [44]. Proline accumulation has been correlated with stress tolerance and its concentration is generally higher in stress tolerant than in stress sensitive plants [45]. Likewise, phenolic compounds are very important plant constituents because their hydroxyl groups confer scavenging ability [46]. Phenolic compounds in leaves have received considerable attention because of their potential antioxidant activities [47]. Therefore, we concluded that A. lebbeck is partly, more drought resistance than M. azedarach (Tables 7 and 8).
In conclusion, this study shows that the moderate drought stress treatment with amending the planting soil by humic acid, followed by compost, was seems as the optimum condition to obtain appreciable growth for Albizia lebbeck and Melia azedarach. At the same time by following this application, we could save 25% of water consumption to irrigate these transplants.