Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Research Article - (2022)Volume 11, Issue 3
Objective: Building on previous work demonstrating greater severity of Obstructive Sleep Apnea (OSA) in children in winter and spring months due to increased Upper Respiratory Infection (URI) and allergens, along with reports of decreased frequency of URIs with universal infection precautions during the COVID-19 pandemic, this study examines seasonality in pediatric OSA and the effects of the current pandemic at a university-level sleep laboratory.
Methods: In this retrospective chart review we included children under 18 years of age without significant medical co-morbidities, who underwent Polysomnography (PSG) at University of California, Davis sleep lab. We compared the variability in Apnea Hypopnea Index (AHI) between the seasons and compared the demographics, PSG parameters, seasonal variation in AHI between the pre-pandemic (December 2017-March 2020) and pandemic (March 2020-September 2021) periods.
Results: Of the 625 studies, 423 pre-pandemic and 202 pandemic studies, there were no differences in the total number of OSA cases, number of mild to severe OSA cases or obstructive AHI variability between seasons in years before or during the pandemic. Multivariate analysis demonstrated that obesity and age less than 5 years have a significant association with total obstructive AHI over seasonal or pandemic timing.
Conclusion: Seasonal pattern did not exist in our referred population either before or during the pandemic. Delaying surgical intervention for retesting in a favourable season may not be warranted based on our study results. Obese children and children less than 5 years should continue to be referred for PSG on suspicion of OSA.
Pediatric Obstructive Sleep Apnea (OSA); COVID-19; Polysomnography (PSG); Seasonality; Upper Respiratory Infections (URI); Apnea Hypopnea Index (AHI)
The COVID-19 pandemic has proven to be one of the most formidable global public health crises of the 21st century. It has had far-reaching ripple effects that are continuing to be uncovered. First declared a pandemic by the WHO on March 11, 2020, when the global number of cases and deaths were 118,000 and 4,291, respectively [1], the virus has quickly spread with the weekly global report of the European Centre for Disease Prevention and Control (ECDC) as of February 24, 2022 estimating 424,971,978 cases and 5,898,291 deaths [2]. After the pandemic declaration by the WHO, various countries instituted lockdowns designed to mitigate the transmission of the virus by restricting people’s movements. In the United States, California was the first to order a lockdown on March 19, 2020 [3] with many states subsequently following suit.
Pandemic control measures have consequently had some beneficial health impacts such as fewer hospitalizations in the pediatric population from respiratory infections [4-7]. Social distancing, virtual learning for children, and parents/guardians working from home with decreased peer exposure likely have contributed to reduced transmission of respiratory infections and disrupted the usual seasonal pattern associated with them. Conceivably, this phenomenon is also disrupting other usual patterns of disease that are exacerbated by respiratory infections.
Pediatric Obstructive Sleep Apnea (OSA), a prevalent disorder characterized by episodes of upper airway obstruction during sleep that results in hypoxemia and hypercapnia [8], is one such condition that may be worsened by respiratory infections. It is estimated that OSA occurs in 1% to 5% of children, with adenotonsillar hypertrophy as the most common cause [9]. Studies conducted prior to the COVID-19 pandemic have demonstrated that more severe OSA is diagnosed via Polysomnography (PSG) in the winter and early spring months, suggesting that there is seasonal variability in pediatric OSA [10-12]. Authors suggest that during these months, recurrent upper respiratory infections as well as subsequent lingering inflammation may result in adenotonsillar hypertrophy and therefore drive the observed seasonal differences. We aimed to examine the seasonality in pediatric OSA and whether it persisted during the COVID-19 pandemic. We hypothesized that there would be less variation in the severity of OSA in children during the pandemic compared to the variation in severity in years prior to the pandemic due to the decreased incidence of upper respiratory infections in children during the pandemic.
This study is a retrospective cohort study conducted within University of California, Davis (UCD) and approved by the UCD Institutional Review Board (IRB ID: 1733442-2). Medical records of patients under 18 years of age who were referred to the UCD Health Sleep Disorders Center for Polysomnography (PSG) during the period of December 2016 to September 2021 for suspected OSA were analyzed. We excluded children with known genetic syndromes, craniofacial anomalies, neurologic disease, upper airway anomalies, chronic cardiopulmonary diseases except for asthma, recent upper airway infection within 2 weeks of PSG, and those who underwent adenoidectomy and/or tonsillectomy up to one year prior to PSG, similar to prior studies[11,13]. Continuous Positive Airway Pressure (CPAP) titration studies and other non-diagnostic PSG studies were excluded. Although California issued its stay-at-home order [3] on March 19, 2020, some Northern California school districts closed in early March due to increasing cases of COVID-19 among the student body [14,15]. Additionally, the Governor’s stay-at-home order was lifted on June 15, 2021[16] and Northern California school districts largely returned to in-person instruction in fall 2021 following California Department of Public Health guidance [17]. Therefore, the pre-COVID control period was defined as dates before March 1, 2020, and the post-COVID period started on March 1, 2020, until September 2021. Seasons were defined according to the meteorological definition for the Northern Hemisphere: spring, March to May; summer, June to August; fall, September to November; winter, December to February.
Demographic data
Data was collected from electronic health records (EMR-Epic) on race/ethnicity (self-reported), age, and gender. Anthropometric measurements, taken within six weeks of the PSG including height and weight were also included in the study. Body Mass Index (BMI) for children above 2 years, as well as weight-for- length/height for children younger than 2 years of age were calculated based on the World Health Organization (WHO) standards. Obesity for children 2 to less than 18 years was defined as BMI percentile greater than 95% using the CDC growth charts and obesity for children less than 2 years was defined as weight- for-length percentile greater than 95% using the WHO growth charts. Age group was further stratified as under 5 years of age and 5 years of age and older. This is because the pre-school age group is particularly vulnerable to recurrent upper airway infections [18] and the older school-age population has different social exposures. Data was also collected on co-morbid conditions such as asthma, prematurity, allergic rhinitis, and medications such as montelukast or nasal steroids commonly used for allergic rhinitis and OSA. The demographic data were compared between the pre-and post-COVID studies.
Polysomnography (PSG) data
Data was collected from the PSG database (Cadwell Easy 3, Washington, US) from the sleep laboratory. Sleep staging, scoring of arousals, awakenings, apneic and hypopneic events were performed per standard criteria [19]. The following features in each PSG were evaluated: Total sleep time, sleep latency, sleep efficiency, proportion of time spent in different sleep stages including Non-Rapid Eye Movement (NREM) and Rapid Eye Movement (REM), arousal index, Apnea Hypopnea Index (AHI), REM AHI, Central AHI, and end-tidal CO2 (ETCO2) or transcutaneous CO2 (tcpCO2). CO2 retention was defined as greater than or equal to 25% of sleep spent in ETCO2 or tcpCO2 more than 50 mmHg. OSA severity was defined as follows: normal, AHI<1; mild, 1>AHI>5; moderate, 5>AHI>10; severe, AHI>10 [20]. PSG studies with technical flaws or inadequate data were also excluded. If a patient had more than one test, then only the most recent diagnostic PSG prior to surgery (if applicable) was included. PSG parameters were compared between the seasons in both pre-and post-COVID periods. PSG parameters were also compared between younger (age less than 5 years) and older children.
Statistical analysis
Continuous clinical and demographic characteristics were reported as a mean with standard deviation, and normal and non-normal distributions were reported as a median with interquartile range. Univariate comparisons were made with Chi- square and Fisher exact (<5) tests for categorical variables and Mann-Whitney U and Kruskal-Wallis for 2 and>2 (i.e., seasonal) non-parametric continuous variables, respectively. Normally distributed continuous variables were analyzed with t-tests. All p values are the product of 2-sided tests with p<0.05 considered statistically significant. Multivariate linear regression analysis was performed for predictors of obstructive total and REM AHI as descriptors of OSA severity. All statistical analysis was conducted with IBM SPSS Statistics for Macintosh, version 27.0 (Armonk, NY: IBM Corp).
A total of 1,452 patients were referred to the UCD Health Sleep Disorders center during the period of December 2016 to September 2021 for clinical suspicion of OSA. 827 patients were excluded for age greater than 18 years (n=11), genetic syndromes (n=248), craniofacial abnormalities (n=49), neurologic disease (n=127), upper airway anomalies (n=69), cardiopulmonary disease not including asthma (n=40), non-diagnostic or incomplete studies (n=52), upper airway infection within 2 weeks of study (n=20), tonsillectomy within 1 year of study (n=17), and combinations of aforementioned reasons (n=194). This yielded 625 evaluable studies.
Demographic data
Demographic characteristics are presented in Table 1. There was no difference in gender, average age, and BMI between the pre-and post-COVID studies. There was a higher proportion of younger children (children less than five years) presenting for PSGs post-COVID (p=0.013). Regarding ethnicity, there was reduced representation of patients identifying as non-Hispanic and an increased representation of patients identifying as Hispanic after COVID (p=0.019). Obesity in patients referred for PSG was found to be more prevalent after state wide lockdown (p=0.045). The usage of medications (nasal steroids and montelukast), as well as co-morbidities were equally represented between the groups.
| Pre-COVID-19 onset | Post-COVID-19 onset | p-value | |
|---|---|---|---|
| Dates | 12/12/16 -2/28/20 | 3/1/20-9/11/21 | |
| N | 423 | 202 | |
| Demographic characteristics | |||
| Age | 6.5 [4.0,10.3] | 6.3 [3.4,10.8] | 0.285m |
| Age<5 years (n,%) | 135 (31.9) | 85 (42.1) | 0.013c |
| Gender (n,%M) | 236 (55.8) | 122 (60.3) | 0.276c |
| BMI | 19.8 ± 6.6 | 20.6 ± 7.1 | 0.163t |
| Ethnicity (n,%) | 0.019c | ||
| Not Hispanic/Latino | 308 (72.8) | 131 (65.2) | |
| Hispanic/Latino | 100 (23.6) | 53 (26.4) | |
| Unknown/declined to state | 15 (3.5) | 17 (8.5) | |
| Co-morbidities | |||
| Obesity (n,%) | 104 (24.7) | 65 (32.3) | 0.045c |
| Prematurity (n,%) | 36 (8.5) | 19 (9.4) | 0.718c |
| Rhinitis (n,%) | 144 (34.0) | 63 (31.2) | 0.478c |
| Asthma (n,%) | 130 (30.7) | 62 (30.7) | 0.992c |
| Medications | |||
| Montelukast (n,%) | 51 (12.0) | 26 (12.9) | 0.772c |
| Flonase (n,%) | 176 (41.6) | 84 (41.6) | 0.996c |
| Sleep characteristics | |||
| Total sleep time | 396 ± 59 | 379 ± 64 | 0.001t |
| Sleep latency | 19.5 [7.0,38.5] | 22.8 [8.8,49.1] | 0.065m |
| Sleep efficiency | 87.2 ± 9.1 | 84.6 ± 11.6 | 0.002t |
| Arousal index | 9.5 [7.3,13.2] | 10.3 [7.4,14.8] | 0.171m |
| OSA severity | 0.411c | ||
| None | 65 (15.4) | 28 (13.9) | |
| Mild | 210 (49.6) | 97 (48.0) | |
| Moderate | 77 (18.2) | 48 (23.8) | |
| Severe | 71 (16.8) | 29 (14.4) | |
| Total obstructive AHI | 3.4 [1.6,6.9] | 3.9 [1.8,7.2] | 0.494m |
| Obstructive REM AHI | 5.1 [2.1,11.8] | 6.8 [2.6,13.2] | 0.162m |
| Central AHI (n,%) | 18 (4.3) | 3 (1.4) | 0.095f |
| Median [IQR] | 2.6 [1.3,4.2] | 4.7 [3.0, n/a] | 0.153m |
| O2 nadir % | 92.0 [89.0,94.0] | 92.0 [88.0,94.0] | 0.252m |
| CO2 retention (n,%) | 44 (10.4%) | 20 (9.9%) | 0.439c |
Note: Median [IQR], Mean ± SD, t=t test, c=Chi-squared test, m=Mann Whitney U test, f=Fisher exact test.
Table 1: Univariate analysis of patient characteristics before and after the onset of the COVID-19 pandemic.
PSG parameters for pre-and post-COVID periods
PSG parameters of sleep latency, arousal index, total obstructive AHI, obstructive REM AHI, O2 nadir, central AHI, were similar in pre-and post-COVID periods. Total sleep time and sleep efficiency were, however, found to be reduced in the post-COVID period (p=0.001 and p=0.002, respectively).
Of the 625 PSGs studied, 93 were diagnosed with primary snoring (no OSA), 307 with mild, 125 with moderate, and 100 with severe OSA. There was no difference in frequency of studies positive for OSA or severity of positive studies across different seasons in either pre-COVID or post-COVID periods (Table 2).
| Spring | Summer | Fall | Winter | p-value | |||
|---|---|---|---|---|---|---|---|
| All patients | Pre-COVID | Total (n) | 97 | 108 | 112 | 106 | |
| Positive (%) | 86.60% | 81.50% | 84.80% | 85.80% | 0.744 | ||
| Severity (%) | 0.909 | ||||||
| Mild | 48.50% | 49.10% | 50.00% | 50.90% | |||
| Moderate | 21.60% | 13.90% | 17.00% | 20.80% | |||
| Severe | 16.50% | 18.50% | 17.90% | 14.20% | |||
| Post-COVID | Total (n) | 52 | 77 | 44 | 29 | ||
| Positive (%) | 86.50% | 85.70% | 81.80% | 93.10% | 0.597 | ||
| Severity (%) | 0.686 | ||||||
| Mild | 42.30% | 49.40% | 54.50% | 44.80% | |||
| Moderate | 28.80% | 23.40% | 18.20% | 24.10% | |||
| Severe | 15.40% | 13.00% | 9.10% | 24.10% | |||
| Younger | Pre-COVID | Total (n) | 28 | 37 | 36 | 34 | |
| Positive (%) | 96.40% | 91.90% | 91.70% | 88.20% | 0.71 | ||
| Severity (%) | 0.689 | ||||||
| Mild | 53.60% | 40.50% | 44.40% | 38.20% | |||
| Moderate | 14.30% | 27.00% | 22.20% | 35.30% | |||
| Severe | 28.60% | 24.30% | 25.00% | 14.70% | |||
| Post-COVID | Total (n) | 23 | 37 | 10 | 15 | ||
| Positive (%) | 91.30% | 91.90% | 80.00% | 100.00% | 0.386 | ||
| Severity (%) | 0.295 | ||||||
| Mild | 39.10% | 51.40% | 70.00% | 33.30% | |||
| Moderate | 30.40% | 27.00% | 10.00% | 33.30% | |||
| Severe | 21.70% | 13.50% | 0.00% | 33.30% | |||
| Older | Pre-COVID | Total (n) | 69 | 71 | 76 | 72 | |
| Positive (%) | 82.60% | 76.10% | 81.60% | 84.70% | 0.59 | ||
| Severity (%) | 0.341 | ||||||
| Mild | 46.40% | 53.50% | 52.60% | 56.90% | |||
| Moderate | 24.60% | 7.00% | 14.50% | 13.90% | |||
| Severe | 11.60% | 15.50% | 14.50% | 13.90% | |||
| Post-COVID | Total (n) | 29 | 40 | 34 | 14 | ||
| Positive (%) | 82.80% | 80.00% | 82.40% | 85.70% | 0.968 | ||
| Severity (%) | 0.997 | ||||||
| Mild | 44.80% | 47.50% | 50.00% | 57.10% | |||
| Moderate | 27.60% | 20.00% | 20.60% | 14.30% | |||
| Severe | 10.30% | 12.50% | 11.80% | 14.30% | |||
Table 2: Frequencies of sleep studies positive for OSA and severity of OSA by season and COVID period.
There were no differences in total AHI between seasons in pre-and post-COVID periods (p=0.996, 0.075) nor in REM AHI (p=0.969, 0.073) (Figure 1). Additionally, there were no differences when each season was evaluated individually for pre- COVID and post-COVID variability (Total AHI spring to winter: p=0.530, 0.491, 0.190, 0.113, respectively; REM AHI spring to winter: p=0.110, 0.386, 0.311, 0.144, respectively).
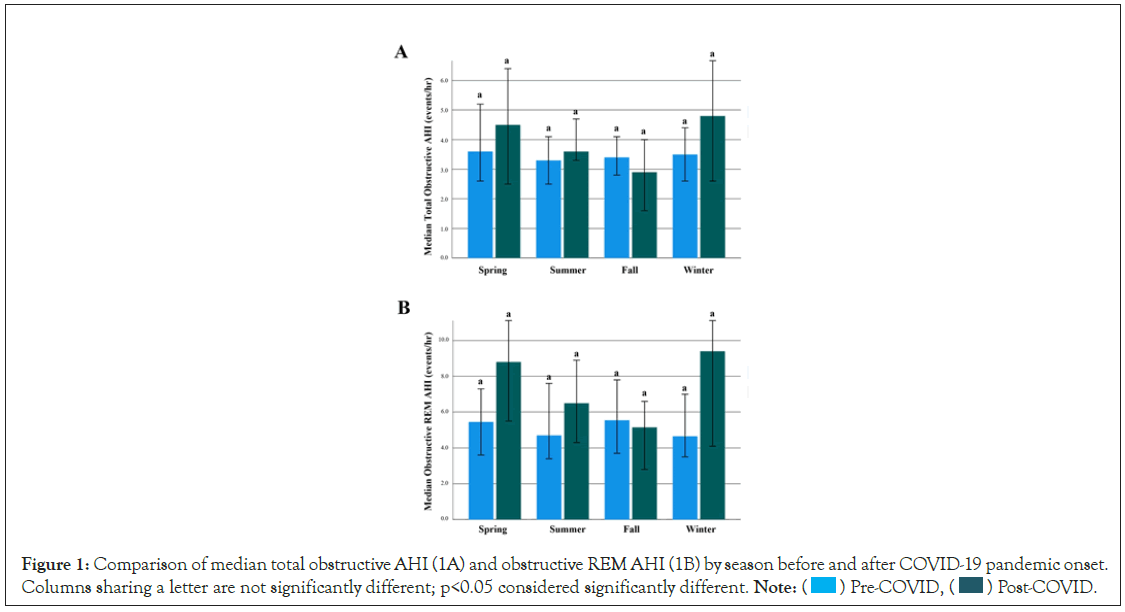
Figure 1: Comparison of median total obstructive AHI (1A) and obstructive REM AHI (1B) by season before and after COVID-19 pandemic onset.
Columns sharing a letter are not significantly different; p<0.05 considered significantly different. Note:  Pre-COVID,
Pre-COVID, Post-COVID.
Post-COVID.
When the study population was divided between younger children and older children, there was no difference for either age group in frequency of studies positive for OSA or severity of positive studies across seasons in either pre-COVID or post-COVID periods (Table 2). There were, however, statistical differences in both total AHI and REM AHI in each season between the age groups (Total AHI-spring to winter: p=0.009, <0.001, <0.001, 0.010, respectively; REM AHI-spring to winter: p=<0.001, <0.001, <0.001, <0.001, respectively) (Figure 2). However, there was not a trend for seasonal variability in neither Total AHI nor REM AHI in either the younger or older children (Total AHI: p=0.848, 0.724, respectively; REM AHI: p=0.515, 0.694, respectively).
Figure 2: Comparison of median total obstructive AHI (2A) and obstructive REM AHI (2B) by season for pre-and school-age children. Columns
sharing a letter are not significantly different; p<0.05 considered significantly different. Note:  Age<5years,
Age<5years,  Age ≥ 5years.
Age ≥ 5years.
Because obesity was represented differently between the pre- COVID studies and post-COVID studies in univariate analysis, BMI was charted over time (Figure 3). There did not appear to be a time-dependent increase in BMI in either younger or older children (R2=0.002, 0.003).
Figure 3: BMI distribution during study ped in pre-and school-age children. Note:  Age<5years, R2 Linear=0.002,
Age<5years, R2 Linear=0.002,  Age ≥ 5years, R2
Linear=0.003.
Age ≥ 5years, R2
Linear=0.003.
Multivariate linear regression analysis was conducted on clinically significant parameters thought to modify the severity of OSA and on parameters found to be distributed differently between pre- COVID and post-COVID studies (Table 3). Obesity and younger children had a significant association with more severe Total AHI and REM AHI. However, neither seasons nor pre- vs post COVID timing had an association with more severe total AHI or REM AHI.
Our study did not suggest a seasonal pattern of OSA in our referred population either before or during the pandemic. Rather, higher severity of OSA was associated with younger children (<5 years of age) and those with obesity, across all seasons and before/ after the onset of the COVID-19 pandemic.
Our results, particularly the absence of seasonal variability pre-COVID, contrast with other studies describing seasonal variability in pediatric OSA [10-12], but are consistent with an institutional study conducted in Israel [13]. It is important to note, however, that these studies, including the present one, were conducted in different climate zones. The present study was conducted at an institution with a referral area covering 65,000 square miles of Northern California where pollen and other allergens are present not only in spring, but also summer and fall [21]. Seasonal allergies can impact sleep and sleep disordered breathing [22]. In addition, a significant number of the children referred to our center were on allergy medications such as nasal steroid sprays (40%) and montelukast (10%), which may have blunted the influence of seasonal allergies on OSA. Although common respiratory virus transmission was significantly reduced in the community our institution serves after statewide lockdown [23], exposure to pollen remained a factor that could have increased upper airway inflammation. Northern California has also been experiencing more intense and longer fire seasons that significantly worsen the Air Quality Index impacting respiratory health and sleep quality [24,25]. Though the relationship between air quality from fires and changes in sleep parameters on PSG has not been established, it is possible that these factors exert a perennial pressure on AHI and potentially negate any seasonality of OSA that may be observed in other climates.
Furthermore, we did not observe any seasonal variability after categorization of the referred children by age group. Younger children are more susceptible to URIs and recurrent URIs, and lingering inflammation from these infections can lead to significant enlargement of adenotonsillar tissue [18]. The younger cohort demonstrated higher severity of OSA compared to older children across all seasons, similar to other studies [26] but did not demonstrate any seasonal difference in the presence or severity of OSA. Additionally, some day care centers remained open during the California lockdown [27], potentially increasing the risk of ongoing URIs for younger children even during the pandemic. We have observed higher number of younger children presenting to the lab during the pandemic which we could attribute to increased concerns for OSA due to ongoing URI exposure. However, the number of cases diagnosed as well as severity of OSA remained the same across seasons during the pandemic. This increase in number of referrals could be from selective triaging of younger children for PSGs due to their relatively lower risk for COVID related complications. The lack of seasonal influence, even in younger children, shows the importance of other genetic and environmental factors such as subtle craniofacial abnormalities resulting in reduced airway space, passive smoke exposure, poor air quality, as well as exposure to perennial-indoor allergens that are important in pediatric OSA. Future studies controlling for multiple risk factors to appropriately assess the influence of seasons on pediatric OSA are still needed. Even so, the current study suggests that postponing surgical intervention to repeat the sleep study in a more favourable season is not needed in these children.
In univariate analysis, obesity was more prevalent in the post- COVID sleep studies (Table 1) although a time-dependent increase in BMI in either pre-school-aged or school-aged children was not observed (Figure 3). Several studies have demonstrated global increased weight gain in children after lockdown measures attributed to reduced physical activity and worsened eating habits [28,29]. In line with previous literature[30,31], obesity was found in multivariate analysis to be a significant predictor of more severe OSA by both total AHI and REM AHI. This underscores the American Academy of Sleep Medicine’s recommendation for PSG referral in obese children with even minimal clinical suspicion for sleep-related breathing disorder [32].
Univariate analysis also revealed statistically significant reduction in TST by 20 min. and sleep efficiency by 2.6% in post-COVID studies when compared to pre-COVID studies. Given that there was not a significant change in AHI in the post-COVID period, reduction in TST and sleep efficiency is unlikely due to obstructive sleep apnea. Instead, this result may be reflective of the increased rates of depression, anxiety, and daytime-naps that have occurred after lockdown, and further studies may be warranted in this regard [33,34]. The pandemic has caused innumerable disruptions to previous lifestyles including isolation, prolonged traumas, and changes to routines, all of which may be linked to the sleep disruptions reported here and globally [35].
In regard to demographics, we observed a difference in the distribution of ethnicity in patients undergoing sleep studies after the institution of the lockdown with an increase in Hispanic/ Latino patients. Although increased health system access when viewed in a vacuum can be seen as favourable, when viewed in the context of a pandemic that has disproportionately affected ethnic minorities, further evaluation is warranted. Reduced patient visits in outpatient clinics throughout most subspecialty departments have been recorded globally. Multifactorial etiologies have been described, including patient reluctance to engage in medical care due to fears of contracting COVID-19 [36-38]. Although multiple complexities preclude us from concluding that our findings are related to reduced reluctance by the Hispanic/Latino community to engage in medical care, literature describing lower levels of knowledge regarding the COVID-19 pandemic among the Hispanic/Latino community should not be ignored [39]. This should serve as a reminder that more work is required to fill the gap in effective public health communication, especially among vulnerable populations.
Three key limitations affected this study. Firstly, the data from this study is sourced from our institutional sleep lab and not from the community, rendering our study with limited generalizability. Additionally, the data consists of sleep studies for patients that were all referred to our institution. This subjects the data to biases that may exist among provider referral patterns. Lastly, our sleep lab limited the access of pediatric patients during the months of March and April 2020, resulting in limited access to sleep studies during these months. This was done to implement a triage system and infection control measures so that our lab could safely maintain accessibility throughout the pandemic. This included appropriate triaging of requests, administering a health screening questionnaire upon arrival, checking vaccination status of patients and families (when available), minimizing the number of patients per night, maintaining 1:1 technician to patient ratio, and enforcing strict COVID precautions for the sleep lab technicians. We have not experienced any outbreaks in our lab due to these measures and the number of PSGs conducted before and during the pandemic was similar. This speaks to the efficacy of these measures maintaining safe accessibility to our lab services.
Our study does not suggest any distinct seasonal trend in OSA severity in our patient population and this trend has not changed during the pandemic. Specific delay of medical or surgical intervention for retesting in a more favourable season may not be warranted. Although an increased BMI trend was not noted to date, the increased rates of reported obesity in the post-lockdown population correlates with anecdotal observation. Because age less than 5 and obesity are shown to modify OSA severity in this study, patients with these risk factors should continue to be referred for sleep studies on suspicion of OSA.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
Citation: Aulakh SS, Funamura JL, Solis RN, Virani FR, Nandalike K (2022) Impact of COVID-19 on Seasonality of Pediatric OSA: An Experience from the University-Level Sleep Lab. J Sleep Disord Ther. 11:369. DOI: 10.35248/2167-0277-22.11.369.
Received: 30-May-2022, Manuscript No. JSDT-22-17674; Editor assigned: 02-Jun-2022, Pre QC No. JSDT-22-17674 (PQ); Reviewed: 16-Jun-2022, QC No. JSDT-22-17674; Revised: 23-Jun-2022, Manuscript No. JSDT-22-17674 (R); Published: 30-Jun-2022 , DOI: 10.35248/2167-0277.22.11.369
Copyright: © 2022 Aulakh SS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.