Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Case Report - (2019)Volume 10, Issue 4
Methotrexate (MTX) has been approved for the treatment of psoriasis and is considered to be effective and relatively safe. This drug was widely used for treatment of psoriasis in Vietnam for many years. Life-threatening MTX toxicity is rare but may rapidly develop when the drug is administered. In line with the interface dermatitis and a remarked eosinophilic infiltration in the cutaneous lesion, the diagnosis of hypereosinophilia as an adverse reaction in our patient was supported by the dramatic improvement of symptoms and decreased eosinophils after withdrawal of MTX. To the best of our knowledge, this is the first case that showed isolated hypereosinophilia as an adverse reaction of MTX in psoriasis treatment that may challenge in early recognition. Awareness and prompt recognition of this severe adverse reaction can result in life-saving discontinuation of MTX.
Methotrexate; Drug adverse reaction; Eosinophilia; Psoriasis
Methotrexate (MTX) has been approved for the treatment of psoriasis and is considered to be effective and relatively safe. Lifethreatening MTX toxicity is rare but may rapidly develop when the drug is administered [1]. Here, we report the first case of hypereosinophilia alone as a side effect of MTX in a psoriatic patient.
A 20-year-old male presented with a six-year history of recurrent and progressive psoriasis vulgaris. Otherwise, he was healthy, with no history of allergies or asthma. Because of erythroderma, he was admitted to the hospital and was treated with MTX 15 mg/week. After 7 days, he was discharged from hospital but he was again admitted due to severe fatigue and intense pruritus soon after only one week (Figure 1a). He had no wheezing or dyspnea. His whole body was covered by a bright erythema with white scales and some small pustules scattered on his legs. MTX was continued at the same dose with folic acid supplementation.
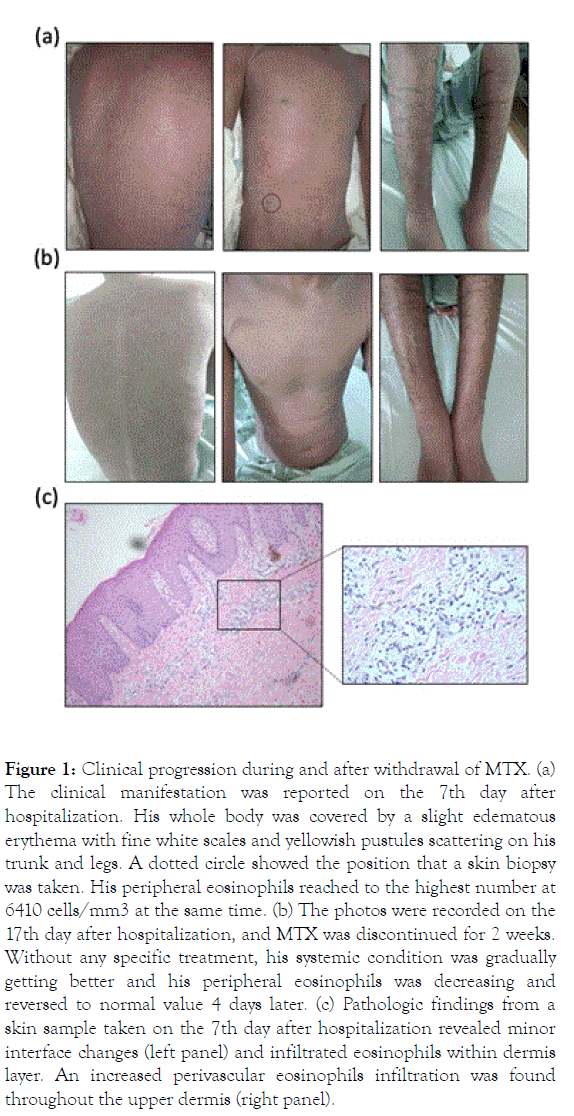
Figure 1. Clinical progression during and after withdrawal of MTX. (a) The clinical manifestation was reported on the 7th day after hospitalization. His whole body was covered by a slight edematous erythema with fine white scales and yellowish pustules scattering on his trunk and legs. A dotted circle showed the position that a skin biopsy was taken. His peripheral eosinophils reached to the highest number at 6410 cells/mm3 at the same time. (b) The photos were recorded on the 17th day after hospitalization, and MTX was discontinued for 2 weeks. Without any specific treatment, his systemic condition was gradually getting better and his peripheral eosinophils was decreasing and reversed to normal value 4 days later. (c) Pathologic findings from a skin sample taken on the 7th day after hospitalization revealed minor interface changes (left panel) and infiltrated eosinophils within dermis layer. An increased perivascular eosinophils infiltration was found throughout the upper dermis (right panel).
Blood tests revealed an elevated numbers of eosinophils at 4630 cells/mm3 (reference range from 0 to 700 cells/mm3). Liver enzymes, renal function tests were all in a normal range. One week later, his condition worsened with fever at 39℃, severe itchy, dark erythroderma and some new pustules on his back (Figure 1b). His peripheral eosinophils were increased to 6410 cells/mm3. Total IgE was increased at 444.4 UI/mL (normal<130 UI/mL). He did not complain of any gastrointestinal discomfort. Blood cultures were negative, chest radiography, hs-CRP and procalcitonin were normal, but a pus culture was positive with S. aureus and linezolide 600 mg twice a day was then added. Serum tests for antibodies against Strongiloides (IgG/IgM), Gnatosthoma spinigerium (IgG), Toxocara canis (IgG), and Fasciola spp (IgG) were negative. Scabies infection was excluded by a careful examination and a negative skin scraping. A prick test was not performed due to his generalized edematous erythroderma.
A skin biopsy revealed a psoriasiform epidermal hyperplasia with minor interface changes and remarkably infiltrated eosinophils within the dermis (Figure 1c). The record of his previous hospitalization showed a normal eosinophils count of 280 cells/mm3 before starting MTX. Taken together, he presented a peripheral eosinophilia as well as a tissue infiltration that fulfilled the criteria for hypereosinophilia [2,3]. After discontinuation of MTX, the eosinophil counts rapidly reverted to normal at 70 cells/mm3 within 2 weeks without any specific treatment. His systemic condition gradually improved with fever reduction, itch relief and improvement of cutaneous lesions (Figure 2). We supposed our patient suffered hypereosinophilia alone as a rare side effect of MTX that requires recognizing properly and prescribing appropriate management.
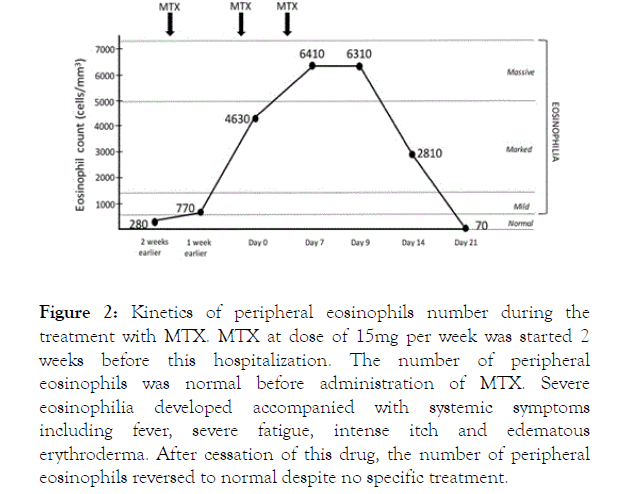
Figure 2. Kinetics of peripheral eosinophils number during the treatment with MTX. MTX at dose of 15mg per week was started 2 weeks before this hospitalization. The number of peripheral eosinophils was normal before administration of MTX. Severe eosinophilia developed accompanied with systemic symptoms including fever, severe fatigue, intense itch and edematous erythroderma. After cessation of this drug, the number of peripheral eosinophils reversed to normal despite no specific treatment.
Blood eosinophilia has been divided into mild (500-1500 cells/mm3), marked (>1500 cells/mm3), and massive (>5000 cells/mm3) eosinophilia [2]. Isolated eosinophilia during MTX treatment is extremely rare, with only three reported cases; two with juvenile idiopathic arthritis and one with rheumatoid arthritis [4]. Based on the compatible of interface dermatitis with a remarked eosinophilic infiltration can be found in the setting of acute mucocutaneous MTX toxicity [3,5], the diagnosis of hypereosinophilia as a drug adverse reaction was also supported by the dramatic improvement of symptoms as well as decreased number of eosinophils after withdrawal of MTX. To the best of our knowledge, our patient was the first case that showed nonspecific clinical manifestations related to a hypersensitive drug reaction including an intense pruritus, severe fatigue and flare of psoriatic lesions that may challenge in early recognition [6].
The underlying of hypereosinophilia due to MTX is still largely unknown. MTX induces apoptosis selectively in activated, proliferating CD4+ T-cells at six-fold higher rates than in resting T cells. In a case of severe psoriasis, we speculate that the robust suppression of Th1 and Th17 activity due to MTX induces a secondary imbalance of the immune system that subsequently leads to reactive eosinophilia through the Th2 downstream pathway. Awareness and prompt recognition of these adverese reactions can result in life-saving discontinuation of MTX.
None declared.
Citation: Le TTV, Nguyen CTH, Nguyen HT, Tro CV, Tran GH, Nguyen TB, et al (2019) Hypereosinophilia as a Rare Side Effect of Methotrexate in a Patient with Erythrodermic Psoriasis: The First Case Report. J Clin Exp Dermatol Res. 10:501. doi: 10.35248/2155-9554.19.10.501
Received: 04-Jul-2019 Accepted: 17-Jul-2019 Published: 24-Jul-2019 , DOI: 10.35248/2155-9554.19.10.501
Copyright: © 2019 Le TTV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : None