Journal of Hepatology and Gastrointestinal disorders
Open Access
ISSN: 2475-3181
ISSN: 2475-3181
Research Article - (2021)Volume 7, Issue 4
Background: Accurate HER2 status is crucial for Gastric Adenocarcinoma (GC) patient selection for antiHER2 therapy. It is assessed Immunohistochemically (IHC) for protein expression, and by Silver In-Situ Hybridization (SISH) for gene copy number. This study aimed to evaluate the concordance of HER2 status by IHC/SISH analyses and HER2-SISH based survival.
Methods: This prospective study includes 145 GC’s (excluding gastro-oesophageal-junction tumours) from the National Hospital of Sri Lanka with defined demographic, clinical-radiological-pathological characteristics. HER2- IHC was assessed by DAKO A0485, RealTM Envision system and interpreted using Ruschoff criteria. HER2-SISH was assessed with INFORM HER2 dual ISH DNA Probe Cocktail. Concordance between HER2 IHC/SISH results was determined by Cohen’s kappa statistics. The association between the survival and HER2-SISH positivity was evaluated using the cox-regression method. Adjustments were done for age, gender, Lauren classification, tumour location and the tumour staging.
Results: The 69 gastrostomies and 76 biopsies, 8.3% (n=12) were HER2-IHC positive (n=7, +2 and n=5, +3). HER2- SISH positivity was 4.8% (n=7). All IHC+3 were SISH positive, while two +2 cases were SISH positive. Concordance for IHC 0, +1, +3 were 100%. There was a significant overall correlation (kappa=0.72, p<0.001) between HER2-IHC and HER2-SISH indicating substantial concordance. The mean overall survival of HER2-SISH negative and positive patients were 41.7(0-210) and 14.6(3-51) weeks respectively, after a mean duration of patient follow up for 40.4 weeks (range 0-210). Survival was relatively lower (p=0.001) in the group with HER2-SISH positivity.
Conclusion: HER2-IHC was well concordant with HER2-SISH for 0, +1, +3 scores and could be used for treatment and prognostication in low resource settings, where SISH facility is unavailable. HER2-IHC+2, without gene amplification may be due to transcriptional activation by other genes or post-transcriptional events, mandating further evaluation by SISH. Survival of GC patients is significantly affected by HER2-SISH positive status. Core tip-HER2 positive Gastric Carcinomas (GC) are usually associated with more aggressive. Aim of this study is to find out HER-2 positivity based on IHC and SISH in Sri Lankan Gastric adenocarcinomas which was not described previously.
Gastric carcinoma; HER2; SISH; IHC; Survival; Concordance
HER-2: Human Epidermal Growth Factor Receptor-2; GC: Gastric Adenocarcinoma; GOJC: Gastro-Oesophageal Junction Cancer; IHC: Immunohistochemistry; SISH: Silver In-Situ Hybridization; CEP 17: Centromere Portion of Chromosome 17; FFPE: Formalin-Fixed Paraffin-Embedded Tissue Sections; FISH: Fluorescence In-Situ Hybridization; CISH: Chromogenic In-Situ Hybridization.
HER2 positive Gastric Carcinomas (GC) are usually associated with more aggressive biological behavior [1-6]. The Trastuzumab for Gastric Cancer (ToGA) trial revealed trastuzumab in combination with chemotherapy, significantly improves the overall survival of patients with HER2 positive advanced GC or Gastro esophageal Junction Carcinoma (GOJC)6. Based on the results of the ToGA study in 2010, the Food and Drug Administration (FDA) [7] in USA and Therapeutic Goods Administration (TGA) in Australia granted approval for trastuzumab in combination with chemotherapy for the treatment of patients with GC and GOJC. Subsequently this therapy has been approved globally for GC and GOJC [8].
The reported frequency of HER2 protein overexpression by Immunohistochemistry (IHC) in GC ranges from 8.2% to 40.3% [1-6,9-12].Only a single documentation is available from Sri Lanka of HER2 expression by IHC in Sri Lankan GC patients.
HER2 status is determined by Immunohistochemistry (IHC) and In-Situ Hybridization (ISH) techniques. IHC is semi-quantitative, evaluating HER2 protein expression. IHC has the advantage of being relatively inexpensive and is widely used in clinical laboratories on Formalin-Fixed Paraffin-Embedded Tissue Sections (FFPE). In contrast, In-Situ Hybridization (ISH) is quantitative, evaluating the HER2 gene copy number. Three types of ISH methods are used for HER2 status determination; Fluorescence In-Situ Hybridization (FISH), Chromogenic In-Situ Hybridization (CISH) and Silver In- Situ Hybridization (SISH) [13,14]. SISH is the preferred method among these different techniques [15]. HER2-SISH status data of Sri Lankan GC patients is unavailable, with paucity of similar data from South Asia.
Sri Lanka has a low incidence of GC in comparison to global and regional countries, with an incidence of 1.2 per 100,000 population and an age adjusted mortality rate of 6.7 [16]. Current study examined the HER2 status of GC patients in Sri Lanka by IHC and SISH techniques and sought to determine the concordance rate. It also aimed to evaluate HER2-SISH based survival of GC patients who did not receive anti HER2 therapy.
Study setting and ethics
This prospective, collaborative study was conducted at the Departments of Surgery and Pathology, Faculty of Medicine, University of Colombo, Department of Pathology, National Hospital of Sri Lanka (NHSL) and the Department of Anatomical Pathology, Pathwest QE II Medical Centre, Perth, Australia. Ethical approvals were obtained from the ethics committees of the Faculty of Medicine, University of Colombo and the NHSL.
Study population
One hundred and forty-five (145) consecutive GC patients (excluding GOJC cases) presenting to the NHSL over four years (2012 April-2016 April) were studied and followed up until 2017 December. None of these patients received anti HER2 therapy. HER2 expression by IHC, clinicopathological features and survival finding of the initial 100 patients in the cohort have been documented previously [12].
In all patients the diagnosis was confirmed by Upper Gastrointestinal Endoscopy (UGIE) and biopsy. Only the gastric resection specimen was included in patients who underwent curative surgery following biopsy. The endoscopic biopsy was included in patients with advanced tumours, who did not undergo gastric resection. Age at diagnosis, gender, type of specimen, tumor location (proximal/ distal stomach) and radiological stage assessed by contrast enhanced computerized tomography (CECT) of the abdomen and thorax were documented using a structured data sheet. Radiological data was used to determine the T (tumor), N (nodal with enlargement >1 cm) and M (metastasis) stages of patients who only had biopsies without resections. Pathological data were used to determine the T and N stages in patients who underwent resections. The TNM stage was determined in accordance with the seventh edition of the UICC guidelines [17].
Pathology
FFPE tumour samples were stained with haematoxylin and eosine. Lauren’s classification for GC was used for histological typing (diffuse, intestinal or mixed) [18].
Immunohistochemistry
FFPE tumour tissue sections were stained for HER2 protein expression using polyclonal rabbit anti-human c-erB-2 oncoprotein (Dako A0485) and Dako Real TM Envision systems. Breast cancer tissue with a HER2-IHC +3 score was used as the positive control. According to Ruschoffscoring [19] a score of IHC 0 or +1 was considered negative for HER2 overexpression, whereas a score of IHC +3 was considered strongly positive. A score of IHC +2 was also considered positive for HER2 overexpression based on criteria by Ruschoff et al.
Silver in-situ hybridization
Tissue Microarrays (TMAs) were prepared at the Department of Anatomical Pathology, Pathwest QE II Medical Centre, Perth, Australia, using two tissue cores with a diameter of 0.6 mm extracted from each tumor using the TMA arrayer (TMA Master 1.16 SP1). Each TMA also contained various non-gastric tissue samples as controls. Sections of 4 μm obtained from the TMA blocks were used for SISH/IHC. The slides were stained using automatic staining devices the Benchmark Ultra view (Ventana Medical Systems).
The INFORM HER2 dual ISH DNA Probe Cocktail was used for HER2 SISH as the probe. This was designed to quantitatively detect amplification by light microscopy of the HER2 gene and the centromere portion of chromosome 17(CEP17), via two colour chromogenic In-Situ Hybridization (ISH) in FFPE GC tissue, following staining on Ventana automated slide strainers. During SISH signal counting, a discrete signal was counted as a single copy of HER2 or CEP17. HER2 SISH signals (black) are typically smaller in size and more discrete in appearance than CEP17 SISH signals (red), due to differences in target sizes and detection chemistries. During the signal interpretation process 20 cells were counted for red (CEP 17-Chromosome 17 centromere) and black (HER2 signals). HER2 gene status was classified as non-amplified (HER2/ Chr17 ratio <2.0) or amplified (HER2/Chr17 ratio ≥ 2.0).
Statistics
The statistical software program SPSS 21(SPSS Inc., Chicago, IL, USA) was used for data analysis.
Evaluation of HER2-IHC vs. HER2-SISH
The concordance between HER2-IHC and HER2-SISH was determined by using Cohen’s kappa statistics.
Survival analysis
Initially survival analysis was performed by the Kaplan-Meier method with log-rank test comparing the survival of HER2-SISH positive and negative groups. Subsequently the association between the survival time and HER2-SISH positivity was evaluated using the cox-regression method. Adjustments were done for age, gender, Lauren classification, tumour location and tumour staging.
Demographic, clinical-radiological-pathological characteristics
IThere were 69 (47.6%) gastric resections and 76 (52.4%) endoscopic biopsies. A male predominance was observed with a male: female ratio of 1.6:1. The mean age at diagnosis was 60.06 (range 32-82 years). Most tumours (n=87, 60%) were located in the proximal stomach and over 60% of both proximal and distal GC presented at an advanced radiological stage (stage III/IV). Resected gastric specimens were primarily of stage II (n=35, 50.7%) on pathological staging. The majority [72(49.7%)] were of Lauren’s intestinal subtype. Again, majority tumours were moderately differentiated (n=56, 38.6%), while well differentiated, poor differentiated tumours accounted for 38(26.2%) and 51 (35.2%) respectively (Figures 1 and 2).
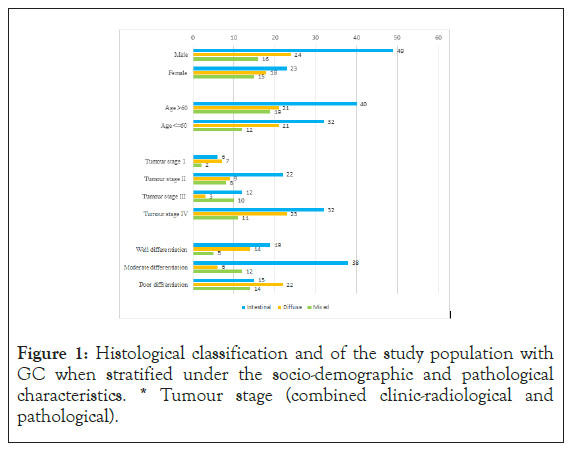
Figure 1: Histological classification and of the study population with GC when stratified under the socio-demographic and pathological characteristics. * Tumour stage (combined clinic-radiological and pathological).
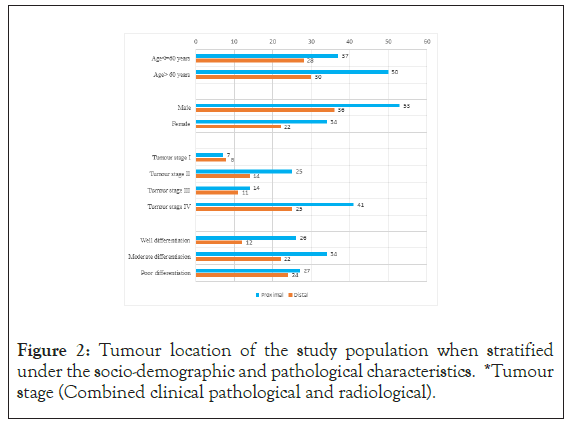
Figure 2: Tumour location of the study population when stratified under the socio-demographic and pathological characteristics. *Tumour stage (Combined clinical pathological and radiological).
There were 8.3% (n=12) HER2-IHC positive cases (Score +2, n=7, Score +3 n=5) HER2-IHC was negative (0, +1) in the majority (n=133, 91.7%) (Figures 3 and 4).
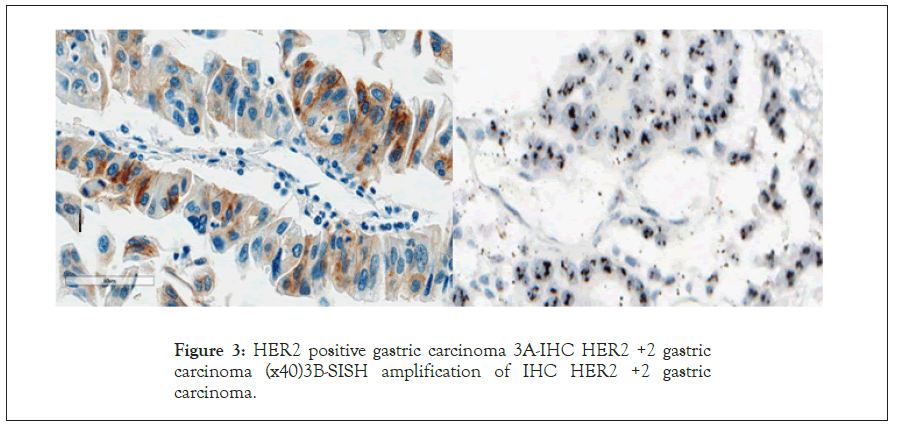
Figure 3: HER2 positive gastric carcinoma 3A-IHC HER2 +2 gastric carcinoma (x40)3B-SISH amplification of IHC HER2 +2 gastric carcinoma.
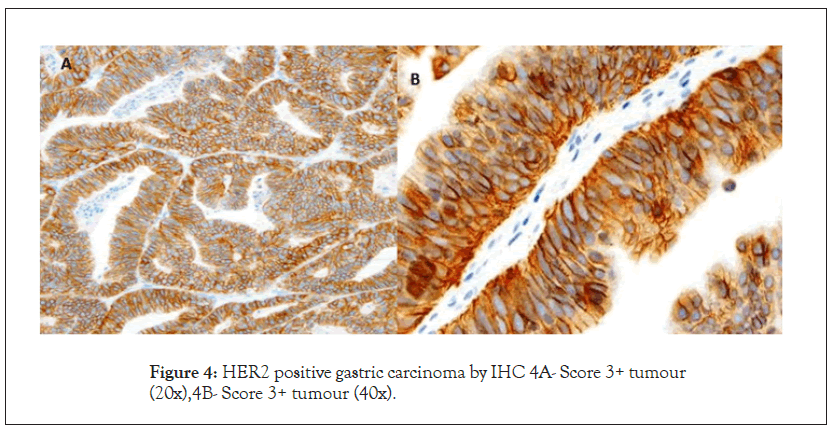
Figure 4: HER2 positive gastric carcinoma by IHC 4A- Score 3+ tumour (20x),4B- Score 3+ tumour (40x).
HER2 by SISH
Table 1 shows the demographic, clinical-radiological-pathological characteristics of HER2-SISH positive and negative GC.
| Demographic/Clinical characteristics | HER2 positive | HER 2 Negative | Total |
|---|---|---|---|
| Age, years | |||
| >60 | 1 | 79 | 80 |
| <=60 | 6 | 59 | 65 |
| Gender | |||
| Male | 7 | 82 | 89 |
| Female | 0 | 56 | 56 |
| Type of specimen | |||
| Endoscopic biopsy | 2 | 74 | 76 |
| Resection | 5 | 64 | 69 |
| Tumour differentiation | |||
| Well | 3 | 35 | 38 |
| Moderate | 1 | 55 | 56 |
| Poor | 3 | 48 | 51 |
| Tumour location | |||
| Proximal | 4 | 83 | 87 |
| Distal | 3 | 55 | 58 |
| Primary tumor | |||
| T1 | 0 | 6 | 6 |
| T2 | 0 | 61 | 61 |
| T3 | 6 | 33 | 39 |
| T4 | 1 | 38 | 39 |
| Regional lymph nodes (radiological/pathological) | |||
| N0 | 0 | 58 | 58 |
| N1 | 7 | 80 | 87 |
| Pathological malignant lymphadenopathy (resections only) | |||
| N0 | 0 | 14 | 14 |
| NI | 3 | 52 | 55 |
| Distant metastases (radiological) | |||
| M0 | 2 | 77 | 79 |
| M1 | 5 | 61 | 66 |
| Radiological/pathological tumor stage | |||
| I | 0 | 15 | 15 |
| II | 3 | 36 | 39 |
| III | 0 | 25 | 25 |
| IV | 4 | 62 | 66 |
Table 1: Comparison demographic, clinical-radiological-pathological features of SISH/HER2 positive and negative GC (N=145).
HER2-SISH was 4.8% (n=7). All HER2-IHC +3 (n=5) cases were SISH positive. Out of the 7, HER2-IHC +2 cases, SISH positivity was observed in 2 cases. All IHC negative cases (0 and +1) (n=133) were confirmed as SISH negative (Figures 3-5).
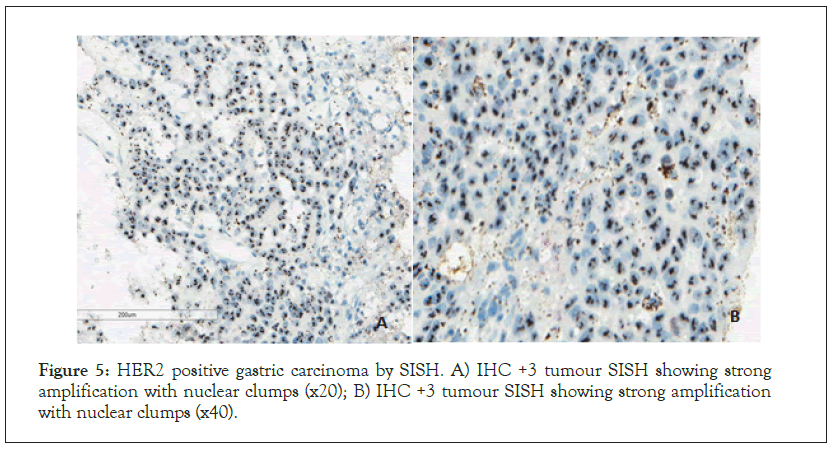
Figure 5: HER2 positive gastric carcinoma by SISH. A) IHC +3 tumour SISH showing strong amplification with nuclear clumps (x20); B) IHC +3 tumour SISH showing strong amplification with nuclear clumps (x40).
Table 2 depicts the concordance between HER2-IHC positivity and HER2-SISH positivity. The reliability of HER2 assessment using IHC and SISH was analyzed using Cohens-kappa statistics. The kappa value was 0.72 (p<0.001), indicating a substantial correlation between HER2 status assessment by IHC and SISH.
| IHC score | SISH amplified | SISH non-amplified | Concordance |
|---|---|---|---|
| 0 (n=124) | 0 | 124 | 100% (124/124) |
| 1+(n=9) | 0 | 9 | 100% (9/9) |
| 2+(n=7) | 2 | 5 | 28.5% (2/7) |
| 3+(n=5) | 5 | 0 | 100% (5/5) |
| Total (n=145) | 7 | 138 | 96.6% (140/145) |
Table 2: Concordance between the results of HER2 IHC and HER2 SISH.
HER2-SISH expression and survival
The mean duration of patient follow up (89% patients for five years or until death as the end point) was 40.4 weeks (range 0-210). All have been treated with standard adjuvant chemotherapy with none receiving anti HER2 (Tratuzumab) therapy.The mean overall survival of HER2-SISH negative and positive patients were 41.7 (0-210) weeks and 14.6 (3-51) weeks respectively. The significantly poor overall survival (p=0.018) of HER2-SISH positive patients is illustrated in Figure 6.
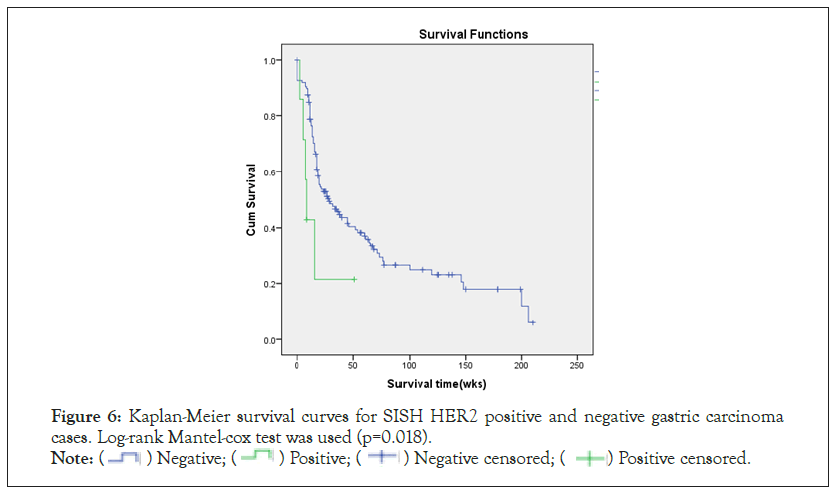
Figure 6: Kaplan-Meier survival curves for SISH HER2 positive and negative gastric carcinoma
cases. Log-rank Mantel-cox test was used (p=0.018).
Note: (  ) Negative; (
) Negative; ( ) Positive; (
) Positive; ( ) Negative censored; (
) Negative censored; ( ) Positive censored.
) Positive censored.
SISH-positivity and advanced age were observed to be independently having a higher hazard ratio for mortality as shown in Table 3.
| Factor/covariate | P-value | Beta coefficient | Standard error | Exponential of Beta OR (CI) |
|---|---|---|---|---|
| SISH-positivity | 0.001 | 2.755 | 0.846 | 15.72 (2.99 to 82.55)* |
| Age | <0.001 | 0.150 | 0.023 | 1.16 (1.11 to 1.21)* |
| Gender | 0.845 | 0.050 | 0.255 | 1.05 (0.63 to 1.73) |
| Lauren Intestinal(I) type | 0.906 | NR | NR | NR |
| Lauren Diffusetype (reference is Lauren I) |
0.989 | -0.004 | 0.299 | 0.996 (0.55 to 1.79) |
| Lauren Mixed type (reference is Lauren I) |
0.663 | -0.151 | 0.345 | 0.86 (0.44 to 1.69) |
| Proximal location (reference is distal location) |
0.341 | 0.243 | 0.255 | 1.28 (0.77 to 2.10) |
| TNM Stage level 1 | 0.977 | NR | NR | NR |
| TNM Staging level 2 (reference is level 1) |
0.659 | 0.270 | 0.611 | 1.31 (0.40 to 4.34) |
| TNM staging level 3 (reference is level 1) |
0.725 | 0.225 | 0.640 | 1.25 (0.36 to 4.40) |
| TNM staging level 4 (reference is level 1) |
0.752 | 0.223 | 0.707 | 1.25 (0.31 to 4.99) |
| Underwent surgery (reference is biopsy for palliative chemotherapy) |
0.207 | -0.687 | 0.545 | 0.50 (0.17 to 1.46) |
Table 3: Associations of variables in the cox-regression with survival.
GC accounts for about 10% of cancer related deaths worldwide with a case fatality rate of 70% [20]. Most (70%) GCs occur in developing countries with half the world’s total cases occurring in East Asia [21,22]. The majority are diagnosed at an advanced stage. Despite multiple therapeutic strategies including radical surgery combined with chemotherapy, improving the outcome of advanced stage GC remains a challenge. In recent years, HER2 receptor status has been identified as a novel molecular targeted therapy for GC [6]. Molecular target therapy for GC depends on the accurate evaluation of the HER2 receptor status or the target gene. Therefore, accurate determination of HER2 status is important for molecular targeted therapy.
HER2 positivity by IHC was 8.2% (n=12), in the study. HER2- IHC has been documented as 9% in the first 100 patients of this patient cohort [12]. IHC +2, +3 were considered as positive in both these studies. While HER2-IHC positivity rates in GC have generally ranged from 8.2% to 40.3% [1-12], Asian studies have reported rates ranging from 11.7% to 15.74% [23-25]. The explanation for this variation is likely to be multifactorial including differences in the populations studied, use of non-standardized assays using different antibodies and the application of different scoring criteria for interpretation. GOJC’s are also reported to have higher HER2 expression rates [26,27]. The GOJC’s were excluded from the current study as these are now considered to be a distinct entity exclusion of GOJC and the use of a polyclonal antibody may have contributed to the lower HER2 positivity rate the study encountered. Alternatively, the low HER2-IHC positivity rate could be a reflection of a genuine difference in tumour biology of the local population or due to heterogeneity of HER2 receptor expression which is a well-recognized phenomenon in GC [28].
HER2 positivity by SISH was 4.8% (n=7) in this study. Four out of 69 resections and 3 out of 76 biopsies showed HER2-SISH positivity. HER2-SISH positivity was more in gastric resections 5.8% (4/69) whereas only 3.9% (3/76) cases of biopsy specimens showed positivity. Lower rates of HER2-SISH positivity in biopsies again may have been due to heterogeneity of the HER2 gene amplification [29].
Most comprehensive data on the concordance between HER2 status by IHC and SISH methods comes from the systematic review and meta-analysis by Pyo et al. [30], which included a total of 12,679 cases from 45 individual studies. Very high concordance rates were found between 0/1+ and 3+ as was expected. Concordance between HER2 IHC2+ and HER2 gene amplification was more variable. The pooled sensitivity and specificity of IHC positivity (when both 2+ and 3+ were considered positive), in predicting ISH confirmed HER2 positivity, were 0.86 and 0.91, respectively. In the current study all cases underwent HER2 amplification testing by SISH. 5 of the 12 IHC+ cases were SISH negative; All 5 SISH negative cases were IHC +2. HER2 gene was amplified in all cases with overexpression of the HER2 protein at +3 level, and HER2 gene was not amplified at 0/+1 level, which is consistent with the reported data [31].Therefore in this cohort of Sri Lankan GC patients HER2-IHC was well concordant with HER2-SISH for 0, +1, +3 scores. These findings are valuable as HER2-IHC could provide useful information in limited resource settings for treatment and prognostication for patients with HER2 scores of 0, +1, +3. This is in keeping with the current recommendation that all patients with GC should have their tumours tested for HER2 status at the time of the initial diagnosis with IHC as the first screening method. Those cases with results considered equivocal for HER2 overexpression (2+) should be referred for HER2 analysis by In-Situ Hybridization [14]. This is endorsed by the study findings were of the 7 IHC +2 cases, SISH positivity was observed in only 2 cases.
The subset of cases showing IHC/SISH discordant results could also be due to heterogeneous HER2 protein expression. Chromosomal instability is probably one of the major causes for this heterogeneity. Kameda et al. detected HER2 overexpression without amplification and considered that this may indicate that gene amplification may not be the primary mechanism by which the HER2 protein is over expressed in GC [32]. HER2 overexpression may occur by a number of different mechanisms, including transcriptional activation by other genes or post-transcriptional events [33]. Therefore, HER2-IHC 2+ cases represent a subset of patients with uncertain diagnostic characteristics in terms of HER2 status. IHC 2+ cases should be interpreted carefully and necessary confirmatory tests for gene amplification should be carried out.
Many studies have examined the hypothesis that HER2 status could be a predictor of the survival rate in patients with GC. Andreas et al. have shown that there is a significant association between the level of HER2 expression and faster achievement of cancerfree and overall survival, without adjusting for the tumour stage [34]. Brien et al. have shown that the pathological stage and HER2 gene amplification are independent prognostic factors of survival in multivariate analysis [35]. According to the ToGA study median overall survival was 13.8 months in HER2 positive GC treated with trastuzumab compared to 11.1 months in the chemotherapy only arm [6]. In keeping with these findings, HER2-SISH positivity and advancing age were associated with a significantly low overall survival in the present study independently of tumour stage and other confounding factors.
HER2 prevalence by IHC and SISH were 8.2% and 4.8% respectively in this Sri Lankan GC patient cohort. The reliability between HER2-IHC and HER2-SISH is satisfactory with substantial agreement. In view of this, HER2 by IHC could be a satisfactory alternative for GC with 0, +1 and +3 scores, in settings where HER2 by SISH is unavailable. Patients with HER2-IHC +2 scores however require confirmatory testing for gene amplification. Additionally, SISH HER2 positivity is associated with a low overall survival among Sri Lankan GC patients who have not received anti HER2 therapy, independently of the tumour stage.
Authors wish to acknowledge the National research council research grant (11-100) for funding the research study and the National Science Foundation travel grant (OSTP/2016/03). Dr Medhavini Dissanayake, Dr Sameera Ravishan, Research assistants, Department of Pathology, University of Colombo, is acknowledged for their assistance in data collection and MrsKokila Wijesinghe, Senior technical officer Department of Pathology, University of Colombo for guidance on IHC staining. Authors also like to acknowledge Mr. Sithum Munasinghe of Translational Health Research Institute, Western Sydney University for his input on statistical analysis.
DS, MDSL, SS, MPK, DNS, AS conceptualized and DS, MDSL, SS designed the work. DS acquired, analyzed and interpreted the data, and wrote the manuscript. MPK and NA helped with Immunohistochemistry work up. MPK helped with supervision on immunohistochemistry interpretation. PKBM helped with Statistical analysis manuscript drafting. All authors read and approved the final manuscript.
This study was funded by National Research Council, Sri Lanka by providing the research grant (11-100). Overseas phase of this study was funded by National Science Foundation for providing me a travel grant (OSTP/2016/03).
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
This study has been approved by the Ethics review committee of the Faculty of Medicine, University of Colombo (EC 11-139) and the Ethics Review committee of the NHSL. All patients gave their written informed consent to collection and use of their data for research purposes. No individual patients can be identified by the anonymous data used in this study.
Citation: Subasinghe D, Acott N, Mahesh PKB, Sivaganesh S, Samarasekera A, Kumarasinghe MP, et al. (2021) Human Epidermal Growth Factor Receptor-2(HER-2) Expression in Sri Lankan Gastric Adenocarcinoma Patients: Analysis of the Concordance of HER2 Expression and Survival Based on Silver In-Situ Hybridization (SISH). J Hepatol Gastroint Dis. 7:193.
Received: 15-Nov-2021 Accepted: 29-Nov-2021 Published: 06-Dec-2021
Copyright: © 2021 Subasinghe D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.