Journal of Pollution Effects & Control
Open Access
ISSN: 2375-4397
ISSN: 2375-4397
Research Article - (2022)Volume 10, Issue 3
The quite effective water treatment technology is also efficient in removing coliform microbes such as E. coli. Water pollution is a serious international issue. Various technologically advanced treatment methodologies, for example, activated sludge process, membrane bioreactor, trickling filter, rotating biological contractor and oxidation ditch are widely studied, well documented and adopted in practice. However, attention to promising low-investment-cost technologies such as slow sand filtration techniques was humdinger miniscule. And bacteria from water/wastewater Apart from reduction of pathogenic load which is ascribed to biological processes SSF can efficiently remove turbidity, suspended solids and toxic metals in treated water. The SSF treated effluent conforms to the discharge standards for potable (drinking) and non-potable (such as irrigation) users realize how hard it is to clean.
Slow sand filter; Wastewater; Wastewater treatment technologies; Water reuse
Clean water is becoming a scarce commodity in many populated areas of the world. People still do not and untreated water is used. Untreated means not conserved, altered or upgraded by the use of a physical, chemical or biological agent. Whereas, untreated water is non-potable water that has been subjected to any process intended to eradicate infection of bacteria, viruses and parasites. These organisms commonly cause illnesses and infect many of the organs of the body. The increment in the population density in the world will cause a high water demand. Unfortunately, sources of clean water have been contaminated by many different human activities. Ordinarily, rural areas have a high body system and the body is also prepared to face any microbes that attack our body. Yet, it is not astute to take all the risk for the whole life All of the usable freshwater in the world, about 97% of it is groundwater. Malaysia (>2%), that means it needs to explore the groundwater sources and not only depend on surface water sources. Mostly, rural communities have their own water supply without any treatment involved. Odors and turbidity are the main causes faced by the rural area communities. Thus, water treatment is a method to purify way of organisms from dangerous organisms. The most effective at a low cost is slow sand filter. However, the knowledge about filtration mechanism still remains limited. Filtration is one of the processes to ensure our clean water is safe from physical pollution. Filtration is the mechanical elimination of turbidity particles by passing the water through a porous medium. The purpose of filtration is to reduce all the turbidity particles carried over from the sedimentation phase, hence producing shimmering clear water with almost zero turbidity. Therefore, this study is to analyze the potential of slow sand filtration(removal efficiency by adding different natural plant bodies, such as, coconut plant fibers, bagasse and oil palm fibers as treatment medium to filter treated water, but with this study we sized different gravels with different sizes such as sand, gravels and medium size particles, so after treatment and before treatment we were conducted physic-chemical characterization by using different water quality measurement parameters like PH temperature, COD,BOD,TDS and other parameters were analysed and to determine the percent efficiency of the slow sand filter to remove E. coli to reduce parameters parameter which is harmful for the rural communities.
Slow sand filtration
The typically slow sand filtration speed of only about 0.55m/hr. At these low rates, the filtered waste contaminants do not penetrate to an appreciable depth within the water treatment medium The filter medium builds up a layer of filtered contaminants (increased height H) on the surface, which at the time being becomes the active filtering medium [1-3].
Rapid sand filtration
Whereas in rapid sand filtration, much higher process velocities were used Filtration occurs through the depth of the filter medium. The comparison between rapid and slow sand filtrating is shown in Table 1. In the United States of America, filter application rates are often expressed as volumetric flow rate per area, or gal/min-ft2, which is actually a velocity with atypical units [4-7].
Sand filtration rates
Table 1 shows the flow rate for both types of sand filter (SSF, RSF).
| Sand filtration type | Velocity of sand filter Rate [m/hr ] |
|---|---|
| Slow Sand filtration | 0.041 to 0.401 |
| Rapid Sand filtration | 0.42 to 3.15 |
Table 1: Range of flow rate both types of sand filter (SSF, RSF).
Filtration process
Most modern sand filtration works on two separated filteration media in the layers. The lower section is composed of a densy material, fine medium, and mostly often sand particles. The upper layer was composed of a less densy particles, large in size medium, mostly often anthracite coal. The large in size upper layer medium were eliminate most larger particles before they reach the fine layer of medium, permit the filtration to operate for a longer period before clogging. As the filteration begins to clog from accumulated solids particle, less water quantities will be passing through it. At some point washing is required. Usual filter operation before cleaning is from a few hours to 1 day. Broadly speaking, filter media should possess the following qualities: such as: Coarse enough to retain large quantities of floc, sufficient fine particles to prevent passage of suspended solids, deep enough to allow relatively long filter runs, and Graded to permit backwash cleaning. These attributes are not compatible. For example, a very fine sand retains floc, which also tends to shorten the filter run, while for a course sand the opposite would be true. Recent trends are toward coarse sands and dual-medium beds of anthracite overlying sand so that high rates of filtration can be obtained. A filtration medium is defined by effective particle size and uniform coefficient. The effective particle size was 11% diameter; that is, 11% by weight of the filtration medium is lower than that particle size diameter. The uniform coefficient is the ratio of the 60% size to the 11% size of particle. In water treatment, the conventional sand medium filtration has an effective size of 0.5-0.6 mm, a uniform coefficient lower than 1.5, and a bed depth (height) of 24-30 in. For both-media filtration, the upper anthracite layer has an effective particle size of 0.8-1.2 mm, a uniform coefficient of lower than 1.8, thickness of a few inches to two thirds of the total filtration thickness of 21-32 in., and is underlain by a sand particle filtration layer as explained above. The supporting large-sized particle sand layer between the filtration sand and the underlying gravel has an effective size of 0.83-2 mm and a uniform coefficient lower than 1.71. The largest particle layer of gravel required is determined by the kind of under drain and size of porosity for movement of filtered and backwashing water [8-11].
Parameters characterization: Its desired water quality measurement parameters include most probably chemical substance, physical substance, and biological matter properties and are measured based on the desired water parameters of concern or its desired for drinking, washing and vegetable supply as a regiation. Parameters that are sampled or monitored for water quality include measures of the following water quality characteristics or parameters, temperature , dissolved oxygen (oxygen in water), PH, conductivity and chloride, turbidity, water level, precipitation (one site). BOD5, COD and other chemical/ physical analysis should be determined.
Physical characteristics of water: Physical characteristics of water such as temperature, color, taste, odor etc. are determined by senses of touch, sight, smell and taste.
Turbidity: Examination Turbidity is a measurement of the percent clarity of water based on the water desired. It is predominantly used for potable water monitoring, although it is infrequently used to assess waste water treatment processes, i.e removal or partial elimination of unwanted particles or matter from the water body. Befogged water is caused by small suspended particles scattering or some instant consuming light. Thus, turbidity is an indirect measurement of the amount of suspended matter in the water. However, since solids of different sizes, shapes, and surfaces reflect light differently, turbidity and suspended solids do not correlate well. Turbidity is normally gauged by an instrument that measures the amount of light scattered at an angle of 90.5 from a source beam. Turbidity is important in potable water because microorganisms attach to suspended particles.
Good level of turbidity: Many drinking water utilities strive to achieve levels as low as 0.1 NTU. The European standards for turbidity state that it must be no more than 4 NTU.
Biological oxygen demand: Nutrient concentration, net primary productivity and diurnal variations of dissolved oxygen in the water were studied under three BOD levels; less than 10, 10– 20 and 20–30 ppm ranges. The results indicated 10–20 ppm to be the optimum BOD range for fish culture in wastewater ponds. When BOD levels are high, dissolved oxygen (DO) levels decrease because the oxygen that is available in the water is being consumed by the bacteria. Since less dissolved oxygen is available in the water, fish and other aquatic organisms may not survive.
Chemical oxygen demand: Chemical oxygen demand is the amount of oxygen taken to chemically breakdown the contaminants in the water, and biological oxygen demand is the amount of oxygen taken to do this biologically through microbes. There are correlated b/n chemical oxygen demand and biological oxygen demand. However, chemical oxygen demands determination from a process is a faster and more accurate method.
Physiochemical composition analysis of waste water (before and after treatment) In table showed the above the water composition after treatment can be reduced the value of components so the treatment is effective up to level of drinking. Then turbidity of waste water we measured 1.2NTU. And treated water from slow sand filter and bacteria disinfected could be measured 0.01NTU. So the value valid is according to European standard (Tables 2 and 3).
| Name of matter | Value of matter available(before analysis)(M/l) | Value of matter available (after analysis)(Mg/l) |
|---|---|---|
| Ca | 30.3 | 9.87 |
| Tds | 321 | 67 |
| Na | 34 | 5 |
| K | 78 | 1.14 |
| Cl | 80 | |
| Bicarbonate Caco3 | 120 | 58.8 |
| Mg | 28 | 4.6 |
| F | 23 | 0.45 |
| Total iron | 2 | 0.03 |
| No3 | 5 | 0.98 |
| Total alkality | 67 | 30.5 |
| PH | 3.0 | 7.1 |
| So4 | 20 | 3 |
Table 2: Chemical analysis of both treated and untreated water.
| Disinfection | Colony in no. | C.F.U |
|---|---|---|
| before | 230 | 2300000000 |
| After | 31.5 | 31500 |
Table 3: Biomass before and after bacteria disinfection.
Note: All chemical analysis with this experiment can obtainedwithin the range of European standard (Figure 1).
Figure 1: Comparison b/n treat and untreated water. 

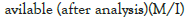
Determination of biological oxygen demand
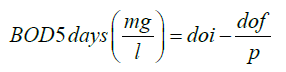
Doi=initial oxygen demand
Dof = final oxygen demand
P= fractional w/w sample to total combined vol.
Where the dilution of water is seeded
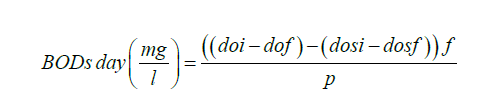
Where ffraction of seeded dilution water in sample to volume of seeded dilution water
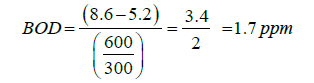
BOD5 day level of 3-5 ppm is considered moderately clean. (Europe standard)
Biological treatment of bacterial disinfection
In this study we used sunlight for bacteria disinfection solar disinfection uses the sun’s ultraviolet (UV) rays to kill microorganisms in water. When a sealed, clear container of water is exposed to sunlight, the UV radiation destroys bacterial, viral, and parasitic pathogens (Figures 2 and 3).
Figure 2: Bacteria disinfection by using sunlight (solar method).
Figure 3: Bacteria counting by using serial dilution.
Bacteria counting (serial dilution)
Serial dilution: 54 ml (9 ml for each test tube) of distilled water was added into 6 test tubes to dilute the sample. The prepared distilled water was foiled with aluminum and then sterilized at 121oC for 15 psi and for 1hrs the sterilized distilled water was then cooled. Then serial dilution was done. Then 54 ml of distilled water were shared for 6 test tube up to 9 ml of distilled water then 1 ml of samples filtrate of caffeine were taken and added into first distilled water test tube label 10-1 using pipette to dilute the sample. Then 1 ml droplet of solution was taken from each of the 1st test tube, and added to each 2nd test tube label 10-2 This procedure was continued up to the 6th test tube of sterilized distilled water for each sample one after the other. 0.1 ml of diluted sample was taken from each of 12 test tubes and inoculated into labeled agar plate (inoculation of the sample occur after agar solution were solidified on plate. This solidification processes may be take up to 20min) then by using sterilized spreader the sample was distributed. Finally, viable cell counted the bacteria can be counted by calculating C.F.U. i.e. Colony Forming Unit.
C.F.U.= no,of colonies/inoculums size (ml)X dilution factor C.F.U/ml
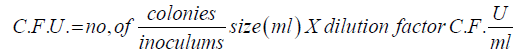
Colony-forming unit (CFU) is a measure of viable bacterial or fungal cells. Before disinfection suppose the plate of the 10^6 dilutions yielded a count of 230 colonies. Then, the number of bacteria in 1 ml of the original sample can be calculated as follows:
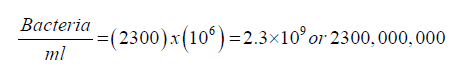
After disinfection suppose the plate of the 10^6 dilutions yielded a count of 30 colonies. Then, the number of bacteria in 1 ml of the original sample can be calculated as follows:
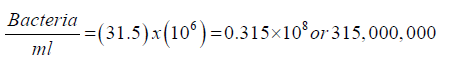
So the table show that after bacterial sunlight disinfection number of bacteria reduced (treated) from the water. So this method can be recommended due to sunlight bacterial disinfection Note. The remained bacteria from bacteria disinfection cannot reproduce (no increase in number) nor harmed to humans when engulf with water because DNA raptured [11-14].
SF was a type of centralized water treatment system. A welldesigned and properly maintained sand filter effectively eliminates turbidity and pathogenic organisms through various biological, physical and chemical methods in one treatment step. Only because of the prevalence of significantly high turbidity or algae pollution, pre-treatment measures (sedimentation) become necessary. Sand filtration methods are characterized by high reliability and cheaper costs. Moreover, neither installation nor operating and maintenance require more skills. Hence, sand filtration is a promising filtration method for small to mediumsized, rural societies with a fairly good quality of the beginning surface water source. As illustrated by the WHO standard, sand filtration provides a simple but most effective and considerably cheap material that can give positive feedback to a sustainable water management technology system.
[Crossref] [Google Scholar] [PubMed]
Citation: Awol ZS, Abate RT (2022) Household Waste Water Treatment by Using Sand Filter Techniques for Ethiopian Peopleâ??s Quality Drinking Water Supply. J Pollut Eff Cont. 10:336.
Received: 02-May-2022, Manuscript No. JPE-22-16598; Editor assigned: 05-May-2022, Pre QC No. JPE-22-16598 (PQ); Reviewed: 23-May-2022, QC No. JPE-22-16598; Revised: 30-May-2022, Manuscript No. JPE-22-16598 (R); Published: 06-Jun-2022 , DOI: 10.35248/2375-4397.22.10.336
Copyright: © 2022 Awol ZS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.