Short Communication - (2017) Volume 3, Issue 3
Histoplasmosis in Pulmonary Infection Patients from Hospitals in Hanoi, Vietnam
*Corresponding Author: Hoang Thi Thu Ha, Bacteriology Department, National Institute of Hygiene and Epidemiology, 1-Yersin Street, Hanoi, Vietnam Email:
Abstract
Histoplasmosis, a fungal infection acquired from soil contaminated with birth/bad or chicken droppings, caused by Histoplasma capsulatum (H. capsulatum), has a worldwide distribution, but is not well studied in Vietnam. While infections are usually asymptomatic, the symptoms of acute or epidemic histoplasmosis are high fever, cough, and asthenia. Immunocompromised patients are at highest risk groups of infection (e.g., persons with cancer, transplant recipients, persons with HIV infection). In this study histoplasmosis has been identified from patients with pulmonary infections using an indirect ELISA (HistoplasmaDxSelect™ kit-USA). Histoplasma capsulatum antibodies were detected in 18% (26/144) of patients. Among these, 57.7% (15/26) were HIV positive and 65.4% (17/26) were chronic pulmonary infections. Histoplasma could not be isolated from patient samples, however, nine cases were detected from clinical samples using nested PCR and sequencing methods. This is the first report of the presence of H.capsulatum in Vietnam. Further studies, therefore, need to be conducted to determine the actual prevalence of histoplasmosis in Vietnam and to map out endemic areas of the disease in Asia.
Keywords: Histoplasmosis; Pulmonary infection patient; ELISA; PCR; Hanoi
Introduction
Histoplasmosis is a fungal infection caused by Histoplasma capsulatum (H. capsulatum ) a dimorphic fungus that, can affect both humans and animals [1-3]. Most Histoplasma infections are subclinical, however, occasionally disease occurs linked in to the host immune response [4]. The disease is the most common pulmonary mycosis in North America and Central America, but the organism is known to exist in many areas around the world [5-9]. Histoplasmosis usually presents as in immune-compromised individuals with serious underlying disease, including AIDS [10,11]. The occurrence of histoplasmosis in Asia has been recently reviewed with cases of histoplasmosis from India, Malaysia, Singapore, Indonesia, Thailand, South Vietnam and Japan, but the autochthonous nature of the cases reported has not been established unequivocally [8,12,13]. Recently, H. capsulatum was recovered from environmental sources contaminated with bat guano and chicken droppings in Thailand [14]. In Vietnam, two histoplasmosis cases were reported from Southern Vietnam, however, the endemicity of the disease has not been studied. Histoplasmosis is difficult to distinguish from diseases with similar clinical features, such as tuberculosis and bacterial pneumonia. In the laboratory, diagnosis of histoplasmosis is also problematic because the culture-positive rate in patients is usually low, and several weeks are required for H. capsulatum to grow on solid media. In addition, H.capsulatum is a biosafety level 3 pathogen requiring specialist facilities for culturing. Serological methods are faster than culture, but can sometimes generate false positives via cross reaction with other fungal pathogens such as Coccidioidomycosis [3]. Moreover, in cases in which a histopathological diagnosis of histoplasmosis has been made, confusion can occur with other pathogens such as Cryptococcus spp ., or Penicillium marneffei , which can have similar pathologies. Molecular methods such as nested PCR and sequencing are alternatives for rapid detection of Histoplasma .
Here, we aimed to determine the prevalence of histoplasmosis among pulmonary infection patients in Hanoi, Vietnam using different diagnostic methods.
Materials And Methods
Sample collection and preparation for culture
Between August 2012 and January 2013, 206 serum and 158 bronchial washing samples were collected from pulmonary infection patients at Bach Mai and Military 103 hospitals in Hanoi. Criteria for enrolment of patients were at least one of the followings: i) abnormal chest shadow and clinical symptoms such as cough, sputum (haemptylis), or dyspnea; ii) negative mycobacterial examination (smear negative or culture negative). Each patient was asked to provide blood and bronchoalveolar lavage fluid (BALF) sample. Aliquots of the BALF were centrifuged and the supernatant was removed. Sterile PBS was added to the sediments, which were thoroughly mixed before being used to inoculate fungal cultures. Serum was obtained by centrifuging at 2000 g (or rpm) for 10 min at room temperature and was stored at -300°C until analysed.
ELISA
Serum samples were analysed using HistoplasmaDxSelect™ kit (USA) following the manufacturer’s instructions. ELISA results were compared with the reference cut-off OD value reading using an ELISA reader. An index value of >1.10 was used to indicate the presence of antibodies to Histoplasma . An index value of <0.90 indicated that antibodies to Histoplasma were not detected.
Nested PCR
DNA extraction from patient’s samples was performed using QIAamp® DNA mini kits in a 50 μl elution volume following the manufacturer’s instructions. Five microliters of extracted DNA was used for the nested PCR assay. Primers used in the assay to detect H. capsulatum specifically amplified a sequence of DNA coding for a specific portion of the H. capsulatum M antigen gene (Msp1F/Msp2R and Msp2F/Msp3R) (Table 1) [15].
| Primer | Primer sequence | Product size (bp) |
|---|---|---|
| Msp1F Msp2R |
5-aca aga gac gac ggt agc ttc acg-3 5-acc agc ggc cat aag gac gtc-3 |
318 |
| Msp2F Msp3R |
5-cgg gcc gcg ttt aac agc gcc-3 5-ata agg acg tca cga agg gc-3 |
269 |
Table 1: Histoplasma capsulatum PCR specific primers.
Sequencing
The second nested PCR amplicons were purified using the QIA quick PCR purification kit (Qiagen).The purified products were sequenced directly in both directions using a Big Dye Terminator v3.1 Cycle sequencing kit (Life Technologies) in an Applied Bio system 3130 genetic Analyser (Life Technologies). Nucleotide sequences for the 269 bp products were verified using the National Centre for Biotechnology Information, Basic Local Alignment Search Tool.
Culture
One hundred microliter aliquots of each PCR-positive samples were cultured onto Brain Heart Infusion agar (Merck) with 1% glucose. Plates were incubated at 28-30°C for up to 8 weeks. Colonies were identified by microscopic morphology.
Ethical review
The study was approved by the ethics committee of the National Institute of Hygiene and Epidemiology, Hanoi, Vietnam (No. 01 IRB, dated January 6, 2012).
Results and Discussion
Histoplasmosis is a community acquired infection, most often presenting as pneumonia [1]. Although histoplasmosis is not a common cause of lung disease in the tropics, it is probably underdiagnosed due to the technical difficulties of microbiological diagnosis and the likelihood of confusion with tuberculosis. Histoplasmosis diagnosis is usually based on visualization of the fungus in organic fluids (sputum, blood and liquor) or tissues (histopathological evaluation) and by the culture of biological samples. The present study used HistoplasmaDxSelect™ ELISA kit, which utilizes inactivated purified Histoplasma antigen for the qualitative detection of antibodies to Histoplasma capsulatum var. capsulatum in human serum. In this study, a total of 206 serum and 158 bronchial lavage samples were collected from patients with acute or chronic pulmonary infections during 2012-2013 (Table 2). The acute pulmonary group (90/206) was hospitalized with typical symptoms as dry or nonproductive cough, fever, chest pain and fatigue. Distinct patterns were occasionally seen on a chest X-ray. The chronic pulmonary patients (116/206) were commonly in older men who had the clinical manifestations include fatigue, mild fever, night sweats, chronic cough, sputum production and weight loss. Of the 206 patient serum samples tested for antibody reactivity by ELISA, Histoplasma antibodies were detected in 13.1% (27/206) which is high compared with studies form Japan and Thailand [1,5]. Positive samples were not significantly linked to occupational group with 16/206 (7.8%) from farmers, 7/206 (3.4%) from workers and 4/206 (1.9%) from other groups, respectively (Table 3). Presence of Histoplasma antibodies was high in chronic pulmonary patients compared to the acute pulmonary group 15.5% (17/116) and 10% (9/90) respectively. Of the HIV-infected patients 42.8% (15/35) were identified positive with Histoplasma antibodies (Table 3). The higher prevalence of antibodies in farmers might be explained by their close contact to soil and material contaminated with bird/chicken dropping. In Thailand, Pirron et al. showed 90.6% of histoplasmosis patients were Acquired Immunodeficiency Syndrome (AIDS) [5]. However, those patients were positive with H. capsulatum by culture while our patients were cases that diagnosed by clinical signs. In addition, we found 8.74% of chronic pulmonary patients were seropositive, compared with 4.36% with acute pulmonary patients. This could be due to the development of anti-Histoplasma antibodies occurring two to six week’s post-exposure [6]. In acute case where Histoplasma infections is suspected, a second serum, sample should be collected 7-14 days later for testing to avoid false negative results [6]. The serological should be only used as a screening test for evaluation potential exposure to, or infection with, Histoplasma and confirmatory are required.
| Sex | Age (yr) | Occupational | Clinical manifestations | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | 20-40 | 40-60 | Farmer | Worker* | Others** | Acute pulmonary | Chronic pulmonary | HIV*** |
| 119 | 87 | 66 | 140 | 107 | 77 | 22 | 90 | 116 | 35 |
*Worker:
**Other groups include teacher, house worker, officer
***HIV: including acute and chronic pulmonary patients
Table 2: Characteristics of patients included in the study.
| Patients group (n) | Patients positive by ELISA/total number (%) |
Patients positive by ELISA/total number of group (%) |
|---|---|---|
| Acute pulmonary | 9/206 (4.37%) | 9/90 (10%) |
| Chronic pulmonary | 18/206 (8.76%) | 18/116 (15.5%) |
| HIV positive | 15/206 (7.28%) | 15/35 (42.8%) |
Table 3: Comparison of ELISA results in the patients group.
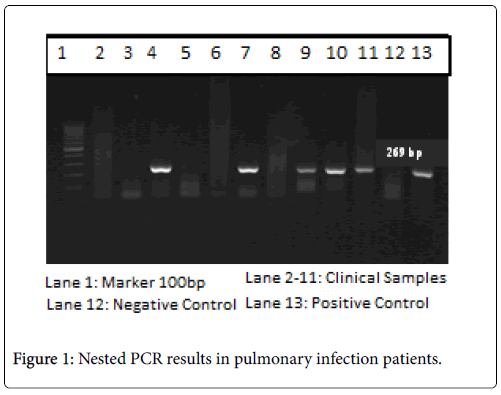
Several reports suggested that PCRs might improve the accuracy of identification of H. capsulatum in clinical samples such as tissues, body fluids. In this study, we used a nested PCR with the specific primers for detection of H. capsulatum in both type of clinical samples. Detail of the primers were previously published but were modified by Ohno H et al. to increase the sensitivity and specificity of PCR allowing for detection of at least 10 fg H. capsulatum DNA [15]. In the present work, 9/206 BALF samples were positive with the nested PCR (Figure 1) and sequence analysis of these PCR products showed approximately 100% similarity to the H. capsulatum M antigen gene. Among these PCR positive samples, 3 of them correlated with a positive ELISA results. While this provided strong evidence that those patients were infected with H. capsulatum , isolation of H. capsulatum from PCRpositive samples by culture was negative after 8 weeks of the incubation period. This could be due to deterioration of the samples during long-term storage or that patients had undergone long-term antibiotic therapy. Therefore, knowledge of clinical syndromes and the patient travel history may lead the physicians to decide sending sample on time and avoid inappropriate treatment regime.
Our results provide evidence that histoplasmosis should be considered in patients with pulmonary infections in Hanoi, Vietnam and that such infections may be under-reported. Physicians should therefore include histoplasmosis in the differential diagnosis of diseases thought to be tuberculosis or community-acquired pneumonia. In addition, diagnosis of histoplasmosis should include culture to allow validation of the non-culture diagnostic technique used in this study in the Vietnam setting. Adaptation and validation of the Histoplasma antigen tests for use in low resource setting could assist with recognition of patients with the infection, allowing early intervention with an appropriate treatment regime.
Acknowledgement
This work was supported by a grant-in-aid of Ministry of Health, Labour and Welfare, the Government of Japan (H23-Shinkoushitei- 020) and Japan International Cooperation Agency (JICA).
References
- Kauffman CA (2007) Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 20: 115-132.
- Bracca A, Tosello ME, Girardini JE, Amigot SL, Gomez C, et al. (2003) Molecular detection of Histoplasma capsulatum var. capsulatum in human clinical samples. J Clin Microbiol 41: 1753-1755.
- Mootsikapun P, Srikulbutr S (2006) Histoplasmosis and Penicolliosis: comparison of clinical features, laboratory findings and outcomes. Int J Infect Dis 10: 66-71.
- O'Sullivan MV, Whitby M, Chahoud C, Miller SM (2004) Histoplasmosis in Australia: a report of a case with a review of the literature. Aust Dent J 49: 94-97.
- Ashbee HR,Evans EG, Viviani MA,Dupont B,Chryssanthou E, et al. (2008) Histoplasmosis in Europe: report on an epidemiological survey from the European Confederation of Medical Mycology Working Group. Med Mycol 46: 57-65.
- Pan B, Chen M, Pan W, Liao W (2013) Histoplasmosis: a new endemic fungal infection in China? Review and analysis of cases. Mycoses 56: 212-221.
- Kauffman CA (2011) Histoplasmosis. Essentials of Clinical Mycology Pp: 321-335.
- Orsi AT, Nogueira L, Chrusciak-Talhari A,Santos M,Ferreira LC, et al. (2011) Histoplasmosis and AIDS co-infection. An Bras Dermatol 86: 1025-1026.
- Kauffman CA (2008) Diagnosis of histoplasmosis in immunosuppressed patients. Curr Opin Infect Dis 21: 421-425.
- Rangwala F, Putcharoen O,Bowonwatanuwong C,Edwards-Jackson N,Kramomthong S, et al. (2012) Histoplasmosis and penicilliosis among HIV-infected Thai patients: a retrospective review. Southeast Asian J Trop Med Public Health 43: 436-441.
- Nhu NTT (2008) Two cases of Histoplasmosis at Cho Ray hospital in 2006-2007. J Med Pharc 7: 1-8.
- Norkaew T, Ohno H,Sriburee P,Tanabe K,Tharavichitkul P, et al. (2013) Detection of environmental sources of Histoplasma capsulatum in Chiang Mai, Thailand, by nested PCR. Mycopathologia 176: 395-402.
- Ohno H, Tanabe K,Umeyama T,Kaneko Y,Yamagoe S, et al. (2013) Application of nested PCR for diagnosis of histoplasmosis. J Infect Chemother 19: 999-1003.
Copyright: © 2017 Ha HTT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.