
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Research Article - (2020)Volume 11, Issue 2
Background: The Acute ischemic stroke (AIS), which results in the corresponding loss of the neurological function, is characterized by sudden losses in the brain of blood circulation. Cardiac troponins (cTns) are used routinely for diagnosing acute myocardial infarction (AMI), but there is a complex overlap between cardiovascular and cerebrovascular disease. The aim of this study was to evaluate the incidence of Hs-cTnT elevation in acute ischemic stroke population and its prognostic significance.
Patients and methods: We enrolled 161 patients admitted with acute ischemic stroke to Menoufia university hospitals and Assalam International hospital. We assessed the full medical history, neurological assessment, National Institutes of Health Stroke Scale (NIHSS), high-sensitive cardiac troponin T (hs-cTnT) level, and ECG on admission and after 24 hours. Statistical analysis was done using R-package for statistical analysis using the appropriate tests.
Results: Our results revealed troponin in admission was elevated above >14 ng/dL in 58% of patients. Troponin dynamicity (delta troponin) defined as 25% increase in the admission value was found in 56% of patients. Analyzing ECG changes and echocardiographic findings revealed significant difference between delta troponin group and static troponin group regarding QTc prolongation (p=0.003), T wave changes (p=0.001), ST segment changes (p=0.013), and reduced ejection fraction (p=0.001). Poor outcome defined in our study as modified ranking scale ≥ 3 was found in 48.4% of patients, and good outcome found in 51.6% of patients.
Conclusion: The acute Hs-cTnT elevation is high in acute ischemic stroke population, Hs-cTnT dynamicity within 24 hours is common, and delta troponin is correlated with stroke severity and poor outcome.
Hs-cTnT; Acute ischemic stroke; Delta troponin; Prognostic value; Static troponin
Stroke is a sudden onset of brain injury with neuronal dysfunction/death caused by an acute focal injury and several pathophysiologic causes [1]. It was estimated that the incidence of strokes worldwide is 17 million annually. Therefore, stroke is considered as the third leading cause of reduced mobility in the world and the fifth leading cause of motility in the United States [2]. Strokes have two types: the most common one is ischemic (85%) and the rest being hemorrhagic type (15%) [3].
Acute ischemic stroke (AIS) is a serious condition characterized by sudden loss of blood supply, oxygen, nutrients, and elimination of metabolic wastes to an area of the brain, resulting in changes obstruct normal neuronal functioning [4]. It was classified into five subtypes according to TOAST trial: 1) Stroke of undetermined etiology, 2) Cardio-embolism, 3) Large artery atherosclerosis, 4) Small vessel occlusion, and 5) Stroke of other determined etiology [5].
Stroke survivors continue to be at higher risk of death, disability, and other serious complications, and it is of great clinical importance to predict outcome in acute stroke. Many biomarkers and clinical variables (such as advanced age and symptoms severity) are suggested as potential predictors of AIS outcomes. However, there is still a great need to identify new biomarkers as predictors for AIS outcomes [6].
Cardiac troponins (cTns) are used routinely for diagnosing acute myocardial infarction (AMI), but there is a complex overlap between cardiovascular and cerebrovascular disease [7]. It was observed that cTns are positive in up to 34% of patients with AIS [8,9]. Till now, there is no clear illustration for this relatively increasing. However, in some patients, raised troponin could reflect an association of coronary artery disease (CAD) and AIS. Moreover, it was suggested that some of the myocardial damage observed in AIS is due to patchy myocyte damage (myocytolysis) due to activation of the sympathoadrenal system that may be linked to insular damage [10].
Cardiac troponins (cTns) were investigated as prognostic markers in the setting of AIS with diverging results. Regarding the association between elevated levels of cTns poor outcomes of AIS, some studies demonstrated a significant association whether cTnT [11,12] and cTnI [13,14], while other studies failed to find an independent association [10,15]. In a previous meta-analysis, they concluded that the elevation in cTns levels in patients with AIS was associated with an overall increased risk of both death and disability [16].
Fortunately, a new generation of highly sensitive troponins (hscTns) assays were developed, which allow for detection of concentrations 5 to 10 times lower than those measurable with conventional assays [17]. In patients with stable coronary heart disease, cTnT measured with a highly sensitive assay at levels below the limit of detection of conventional assays was associated with increased mortality [18]. Furthermore, hs-cTnT provides prognostic information in clinical settings other than acute coronary syndrome, such as severe sepsis, [19] heart failure, [20] and cardiomyopathy. However, the prognostic value of hs-cTnT in the setting of AIS remains to be determined. Therefore, we aim of this study is to assess incidence of hs-cTnT elevation in AIS, its dynamic changes, and the impact of these changes on the morbidity and disability.
Study design
This was a prospective, observational, single group, multicenter study from January 2018 to September 2018 in Menoufia university hospitals and Assalam International Hospital in Egypt. Ethical approval was obtained from the Local Ethics committee of Menoufia university hospitals. Written consent was taken from all patients from themselves or their first-degree relatives. Figure 1 shows the flow diagram of the inclusion process.
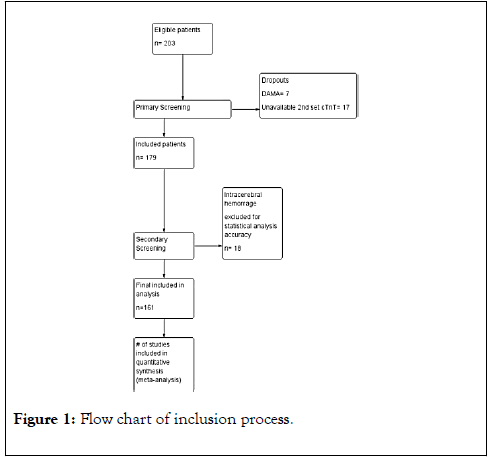
Figure 1. Flow chart of inclusion process.
Inclusion and exclusion criteria
We enrolled all patients older than 18 years admitted to hospital Diagnosed with AIS (clinical and radiological). All patients with the following criteria were excluded from the study:
• Myocardial infarction within one-month preceding study inclusion
• Acute and Chronic renal disease, or hepatic dysfunction
• Acute pulmonary embolism, chronic thromboembolic pulmonary hypertension
• Autoimmune disease
• A significant head injury in the period of the previous 3 months, and a significant injury potentially associated with muscle damage within 30 days prior to study inclusion
• Diagnosed and/ or treated (chemotherapy, radiotherapy) neoplastic disease,
• Documented significant cardiac rhythm disorders except atrial fibrillation, and sudden circulatory arrest requiring defibrillation or external cardiac massage within one-month preceding study inclusion.
Examination and follow-up
All patients enrolled in the study received the following on admission: History data collection related to identified and/or treated cardiovascular and neurological diseases including (hypertension, diabetes mellitus, atrial fibrillation, congestive heart failure, ischemic heart disease, smoking, x-smoking, previous stroke, peripheral vascular disease), Physical examination with assessment of neurological deficit by National Institutes of Health Stroke Scale (NIHSS), Neuro-imaging including non-contrast computed tomography (NCCT brain) or magnetic resonant imaging diffusion weighted (MRI-DW) if available, Hs-cTnT serum level measurement using a highly sensitive assay (Roche Elecsys Troponin Ths, Mannheim, Germany) with an upper reference limit for the 99th percentile of the normal reference population at 14 ng/L and a coefficient of variation of <10% at 13 ng/L, Other laboratory investigations including (hemoglobin level, total leukocytic count, platelet count, international normalized ratio, alanine transaminase, serum cholesterol, serum triglyceride, serum LDL, creatinine, random blood glucose), 12-lead electrocardiogram (ECG), and Hemodynamic data continuously monitored and recorded on admission (systolic blood pressure, diastolic blood pressure, heart rate, respiratory rate).
After 24 hours of admission, all patients were underwent to highly sensitive Troponin T (Hs-cTnT) serum level measurement, Delta troponin was calculated and considered significant if there is an increase in troponin level more than 25% of the baseline value, patients were classified into Dynamic troponin group (2nd troponin value >125% of 1st value) and static troponin group (2nd troponin value <125% of 1st value) and the two groups were compared regarding patient characteristics, admission criteria, labs, ECG changes and echocardiographic findings and delta troponin was correlated to 30 days outcome and mortality, and 12-lead electrocardiogram (ECG).
In the further course of hospitalization, the following was done:
• Transthoracic echocardiography (TTE) using Philips HDX11E
• Need for nasogastric tube, ICU admission and mechanical ventilation
• The modified Rankin Scale (mRS) (21) on the 30th day after the cerebral event to assess poor outcome. Poor outcome was defined as MRS≥3 and good outcome defined as MRS<3
The National Institutes of Health Stroke Scale (NIHSS) is a tool used to objectively quantify the impairment caused by a stroke. The NIHSS is composed of 11 items, each of which scores a specific ability between a 0 and 4. For each item, a score of 0 typically indicates normal function in that specific ability, while a higher score is indicative of some level of impairment. The individual scores from each item are summed in order to calculate a patient's total NIHSS score. The maximum possible score is 42, with the minimum score being a 0 [21,22].
The modified Rankin Scale (mRS) is a commonly used scale for measuring the degree of disability or dependence in the daily activities of people who have suffered a stroke or other causes of neurological disability. It has become the most widely used clinical outcome in stroke clinical trials [23]. The scale runs from 0-6, running from perfect health without symptoms to death.
• 0-No symptoms.
• 1-No significant disability. Able to carry out all usual activities, despite some symptoms.
• 2-Slight disability. Able to look after own affairs without assistance, but unable to carry out all previous activities.
• 3-Moderate disability. Requires some help, but able to walk unassisted.
• 4-Moderately severe disability. Unable to attend to own bodily needs without assistance, and unable to walk unassisted.
• 5-Severe disability. Requires constant nursing care and attention, bedridden, incontinent.
• 6-Dead.
Statistical analysis
Based on previous studies, sample size was calculated using specificity with confidence interval 95% and power of study 80% to be 74 participants. In our study 161 patients were included to increase accuracy and reliability.
Data were collected electronically, tabulated, statistically analyzed using “R Project for Statistical Computing” R version 3.5.2 released 20-12-2018, where the following statistics were applied. Quantitative data were presented in the form of mean and standard deviation (SD), and qualitative data were presented in the form numbers and percentages (%). Chisquared test (χ2) was used to study association between two qualitative variables. Mann-Whitney test is a test of significance used for comparison between two quantitative variables. Receiver Operating Characteristic curve (ROC-curve) was used to determine sensitivity and specificity. p value of (≤ 0.05) was considered statistically significant.
This study included 161 patients with mean age 60.92 ± 13.98 years presenting with AIS. All demographic and clinical data were presented in Table 1.
| Parameters | N (%) / mean ± SD | |||
|---|---|---|---|---|
| Demographic data | Age (mean ± SD) | 60.92 ± 13.98 | ||
| Females | 105 (65.2) | |||
| Comorbidities | Hypertension | 97 (60.2) | ||
| Diabetes | 64 (39.8) | |||
| CAD | 41 (25.5) | |||
| CHF | 14 (8.7) | |||
| AF | 27 (16.8) | |||
| Smoker | 40 (24.8) | |||
| National Institutes of Health Stroke Scale | NISHSS>18 | 19 (11.8%) | ||
| NIHSS (11:18) | 35 (21.7%) | |||
| NIHSS (5:11) | 51 (31.7%) | |||
| NIHSS<5 | 56 (34.8%) | |||
| Stroke classification | LACS | 40 (24.8) | ||
| PACS | 50 (31.1) | |||
| POCS | 50 (31.1) | |||
| TACS | 21 (13.0) | |||
| Hemodynamic and laboratory parameters on admission | SBP | 157.33 ± 18.50 | ||
| DBP | 100.94 ± 11.76 | |||
| HR | 93.39 ± 17.50 | |||
| RR | 21.84 ± 4.44 | |||
| HB | 13.66 ± 1.85 | |||
| TLC | 11.98 ± 28.41 | |||
| CREAT | 1.16 ± 0.75 | |||
| RBS | 203.51 ± 87.82 | |||
| INR | 1.29 ± 1.46 | |||
| SGPT | 34.77 ± 30.42 | |||
| CHOL | 213.37 ± 38.0 | |||
| TG | 157.98 ± 30.96 | |||
| Transthoracic echocardiographic findings | Ejection fraction | EF>60% | 89 (55.3%) | |
| EF 40-60% | 63 (39.2%) | |||
| EF<40% | 9 (5.5%) | |||
| Diastolic Dysfunction | No | 14 (8.7%) | ||
| Grade 1 | 110 (68.3%) | |||
| Grade 2 | 36 (22.4%) | |||
| Grade 3 | 1 (0.6%) | |||
| LA dilatation | 56 (34.8%) | |||
| LVED | 9 (5.6%) | |||
| RSWMA | 20 (12.4%) | |||
| Mitral valve regurge | 22 (13.7%) | |||
| Troponin measurements on admission and after 24 hours | Troponin on admission | 22.79 ± 22.58 | ||
| Troponin 24 | 32.83 ± 30.03 | |||
| Positive troponin on admission | 94 (58) | |||
| Delta troponin | 90 (55.9) | |||
| Poor outcome (MRS≥3) | 78 (48.4%) | |||
| 30 days Mortality | 28 (17.3%) | |||
Data expressed as mean ± SD or Number (percentage) as appropriate CAD: Coronary artery disease, CHF: Congestive heart failure, AF: Atrial fibrillation, Abbreviations: LACS: Lacunar Stroke; PACS: Partial Anterior Circulation Stroke Syndrome; POCS: Posterior Circulation Stroke; TACS: Total Anterior Circulation Stroke; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; HR: Heart Rate; RR: Respiratory Rate; Hb: Hemoglobin Level; TLC: Total Leukocytic Count; Creat: Serum Creatinine Level; RBS: Random Blood Sugar; INR: International Normalized Ratio; ALT: Serum Alanine Transaminase Level; CHOL: Serum Cholesterol Level; TG: Serum Triglyceride Level; LDL: Serum Low Density Lipoprotein Level; EF: Ejection Fraction; LA: Left Atrium; LVEDD: Left Ventricular End Diastolic Dilatation; RSWMA: Resting Systolic Wall Motion Abnormalities
Table 1: Baseline demographics, clinical, laboratory, and troponin measurements of enrolled patients.
Troponin analysis showed that 94 patients (58%) had positive cTnT (>14 ng/dl) on admission and 67 patients (42%) had negative cTnT, mean value of troponin on admission was 22.8 ± 22.5, mean value of troponin after 24 hours was 32.8 ± 30, and 55.9% of patients had dynamic changes in troponin value (25% increase in the admission value). Poor outcome defined as MRS≥3 was found in 78 patients (48.4%) and short-term mortality occurred in 28 patients (17.3%).
Our findings demonstrated statistically significant difference between troponin positive and troponin negative group regarding AF and NIHSS on admission, the positive troponin group had higher prevalence of AF (22%) and more severe stroke on admission NIHSS>18 (17%). Delta troponin (defined as increase >25% of the admission serum level) was found in 90 patients (55.9%). Table 2 shows the comparison between the troponin positive v/s troponin negative groups.
| Variable | Troponin +ve 94 (58%) | Troponin –ve 67 (42%) | p value | |
|---|---|---|---|---|
| Demographic data | Age (mean ± SD) | 61.4 ± 12.3 | 59.1 ± 14.3 | 0.23 |
| Females | 46 (49%) | 59 (88%) | 0.27 | |
| Comorbidities | Males | 48 (51%) | 8 (12%) | 0.12 |
| Hypertension | 55 (58%) | 42 (62%) | 0.35 | |
| Diabetes | 40 (42%) | 23 (34%) | 0.15 | |
| CAD | 28 (30%) | 13 (19%) | 0.095 | |
| CHF | 9 (10%) | 5 (7%) | 0.43 | |
| AF | 21 (22%) | 6 (9%) | 0.019* | |
| Smoker | 14 (15%) | 16 (24%) | 0.48 | |
| Stroke Classifications | LACS | 19 (20%) | 21 (31%) | 0.15 |
| PACS | 36 (38%) | 14 (21%) | ||
| POCS | 28 (30%) | 22 (33%) | ||
| TACS | 11 (12%) | 10 (15%) | ||
| National Institutes of Health Stroke Scale | NISHSS>18 | 16 (17%) | 3 (5%) | 0.001** |
| NIHSS (11:18) | 27 (29%) | 7 (10%) | ||
| NIHSS (5:110) | 27 (29%) | 25 (38%) | ||
| NIHSS<5 | 24 (24%) | 32 (47%) |
Data expressed as mean ± SD or Number (percentage) as appropriate.
*donates statistical significance p<0.05
**donates high statistical significance p<0.001
Abbreviations: CAD: Coronary Artery Disease; CHF: Congestive Heart Failure; AF: Atrial Fibrillation; LACS: lacunar stroke; PACS: Partial Anterior Circulation Stroke; POCS: Posterior Circulation Stroke; TACS: Total Anterior Circulation Stroke
Table 2: Patient characteristics in troponin positive v/s troponin negative groups.
The comparison between static troponin and delta troponin
Our finding demonstrated that the number of patients with hypertension was significantly (p=0.003) higher in delta group (71.1%) compared with static group 46.5%. Moreover, there was a statistically significant difference between the two groups in terms of NIHSS on admission and stroke localization (p<0.001). The delta troponin group had higher incidence of admission severe stroke (NIHSS>18) when compared with static group (15.6% v/s 7%, respectively, p=0.006). Table 3 demonstrates the demographic data, patient characteristics, and clinical picture in static v/s delta troponin group.
| Parameter | Static troponin | Delta troponin | p value | ||
|---|---|---|---|---|---|
| Demographic data | Age | 57.79 ± 13.22 | 63.39 ± 14.14 | 0.011* | |
| Females | 45 (63.4) | 60 (66.7) | 0.789 | ||
| Comorbidities | Hypertension | 33 (46.5) | 64 (71.1) | 0.003* | |
| Diabetes | 27 (38.0) | 37 (41.1) | 0.814 | ||
| IHD | 16 (22.5) | 25 (27.8) | 0.565 | ||
| CHF | 4 (5.6) | 10 (11.1) | 0.346 | ||
| AF | 7 (9.9) | 20 (22.2) | 0.061 | ||
| Smoker | 11 (15.5) | 29 (32.2) | 0.024* | ||
| National Institutes of Health Stroke Scale | NISHSS>18 | 5 (7.0) | 14 (15.6) | 0.006* | |
| NIHSS (11:18) | 9 (12.7) | 26 (28.9) | |||
| NIHSS (5:110) | 24 (33.8) | 27 (30.0) | |||
| NIHSS<5 | 33 (46.5) | 23 (25.6) | |||
| Stroke classification | LACS | 27 (38.0) | 13 (14.4) | 0.001** | |
| PACS | 16 (22.5) | 34 (37.8) | |||
| POCS | 24 (33.8) | 26 (28.9) | |||
| TACS | 4 (5.6) | 17 (18.9) | |||
| Hemodynamic and laboratory parameters on admission | SBP | 157.54 ± 16.32 | 157.17 ± 20.14 | 0.901 | |
| DBP | 101.10 ± 10.89 | 100.81 ± 12.46 | 0.878 | ||
| HR | 90.27 ± 14.90 | 95.84 ± 19.03 | 0.044* | ||
| RR | 21.59 ± 4.22 | 22.04 ± 4.61 | 0.522 | ||
| HB | 13.84 ± 1.64 | 13.51 ± 2.00 | 0.271 | ||
| TLC | 9.57 ± 2.35 | 13.87 ± 37.93 | 0.342 | ||
| CREAT | 8.52 ± 3.71 | 9.67 ± 3.74 | 0.054 | ||
| RBS | 195.03 ± 72.49 | 210.20 ± 98.14 | 0.278 | ||
| INR | 1.10 ± 0.16 | 1.44 ± 1.94 | 0.135 | ||
| SGPT | 29.70 ± 20.52 | 38.77 ± 36.01 | 0.06 | ||
| CHOL | 207.39 ± 41.98 | 218.09 ± 34.05 | 0.076 | ||
| TG | 154.85 ± 28.79 | 160.46 ± 32.51 | 0.255 | ||
| LDL | 104.21 ± 21.11 | 107.44 ± 21.59 | 0.342 | ||
| Transthoracic echocardiographic Findings | Ejection fraction | EF>60% | 49 (69.0) | 40 (44.4) | 0.001** |
| EF 40-60% | 17 (24) | 6 (51.1) | |||
| EF<40% | 5 (7.0) | 4 (4.4) | |||
| Diastolic Dysfunction | No | 50 (70.4) | 60 (66.7) | 0.051 | |
| Grade 1 | 11 (15.5) | 25 (27.8) | |||
| Grade 2 | 0 (0.0) | 1 (1.1) | |||
| Grade 3 | 10 (14.1) | 4 (4.4) | |||
| LA dilatation | 21 (29.6) | 35 (38.9) | 0.287 | ||
| LVED | 5 (7.0) | 4 (4.4) | 0.714 | ||
| RSWMA | 5 (7.0) | 15 (16.7) | 0.11 | ||
| Mitral valve regurge | 9 (12.7) | 13 (14.4) | 0.926 | ||
Data expressed as mean ± SD or Number (percentage) as appropriate.
*donates statistical significance P<0.05
**donates high statistical significance P<0.001
Abbreviations: IHD: Ischemic Heart Disease; CHF: Congestive Heart Failure; AF: Atrial Fibrillation; LACS: Lacunar Stroke; PACS: Partial Anterior Circulation Stroke Syndrome; POCS: Posterior Circulation Stroke; TACS: Total Anterior Circulation Stroke; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; HR: Heart Rate; RR: Respiratory Rate; Hb: Hemoglobin Level; TLC: Total Leukocytic Count; Creat: Serum Creatinine Level; RBS: Random Blood Sugar; INR: International Normalized Ratio; ALT: Serum Alanine Transaminase Level; CHOL: Serum Cholesterol Level; TG: Serum Triglyceride Level; LDL: Serum Low Density Lipoprotein Level; EF: Ejection Fraction; LA: Left Atrium; LVEDD: Left Ventricular End Diastolic Dilatation; RSWMA: Resting Systolic Wall Motion Abnormalities
Table 3: Baseline demographics, clinical, laboratory, and transthoracic echocardiographic findings of Static troponin group vs. delta troponin group.
Echocardiographic studies and ECG finding of both static and delta groups were summarized in Table 4. In terms of complications, 74.4% of delta group required nasogastric tube (NGT) compared to 32.4% of static group (p<0.001).
| Parameter | Static troponin | Delta Troponin | p value |
|---|---|---|---|
| AF1 | 9 (12.7) | 19 (21.1) | 0.177 |
| AF24 | 9 (12.7) | 19 (21.1) | 0.233 |
| Tachy1 | 12 (16.9) | 30 (33.7) | 0.026* |
| Tachy24 | 2 (2.8) | 17 (18.9) | 0.004* |
| QT1 | 26 (36.6) | 52 (57.8) | 0.012* |
| QT24 | 15 (21.1) | 40 (44.4) | 0.003* |
| T1 | 27 (38.0) | 52 (57.8) | 0.02* |
| T24 | 10 (14.1) | 36 (40.0) | 0.001** |
| ST1 | 8 (11.3) | 27 (30.0) | 0.008* |
| ST24 | 7 (9.9) | 24 (26.7) | 0.013* |
| BBB | 1 (1.4) | 4 (4.4) | 0.519 |
Data expressed as Number (percentage) using Chi-square test.
*donates statistical significance p<0.05
**donates high statistical significance p<0.001
Note: AF1: atrial fibrillation on admission, AF24: atrial fibrillation after 24 hours, Tachy1: tachycardia on admission ECG, tachy24: tachycardia after 24 hours in ECG, QT1: QT prolongation on admission, QT24: QT prolongation after 24 hours, T1: T wave changes on admission ECG, T24: T wave changes inn ECG after 24 hours, ST1: ST segment changes on admission, ST24: ST segment changes after 24 hours, BBB: bundle branch block either right or left.
Table 4: ECG changes in delta v/s static troponin group on admission and after 24 hours.
Moreover, there was a significant difference between the two groups regarding requiring mechanical ventilation (p=0.024) and intensive care unit admission (p=0.013). Figure 2 shows the summary of complications in both groups.
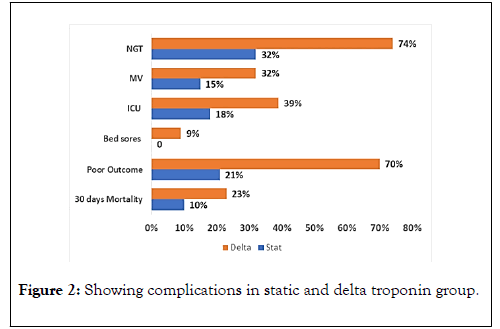
Figure 2. Showing complications in static and delta troponin group.
The comparison between good outcome and poor outcome groups
Table 5 compared poor outcome (defined in our study as modified Rankin scale ≥3) and good outcome groups and showed high statistically significant difference (p<0.001) between good and poor outcome groups regarding age, CAD, AF, NIHSS on admission and stroke localization. Moreover, our findings showed statistically significant difference (p<0.05) in admission parameters between good and poor outcome groups regarding random blood sugar, heart rate, SGPT, Cholesterol, Triglycerides, and LDL.
| Parameter | Good outcome N=83 | Poor outcome N=78 | p value | ||
|---|---|---|---|---|---|
| Demographic data | Age | 56.46 ± (11.09) | 65.67 ± (15.20) | 0.001** | |
| Females | 51 (61.4) | 54 (69.2) | 0.384 | ||
| Male | 32(38.6) | 24 (30.8) | 0.276 | ||
| Comorbidities | Hypertension | 44 (53.0) | 53 (67.9) | 0.076 | |
| Diabetes | 27 (32.5) | 37 (47.4) | 0.077 | ||
| CAD | 10 (12.0) | 31 (39.7) | <0.001** | ||
| CHF | 4 (4.8) | 10 (12.8) | 0.128 | ||
| AF | 5 (6.0) | 22 (28.2) | <0.001** | ||
| Smoker | 17 (20.5) | 23 (29.5) | 0.255 | ||
| Dyslipedemia | 16 (19.3) | 10 (12.8) | 0.369 | ||
| National Institutes of Health Stroke Scale | NISHSS>18 | 0 (0.0) | 19 (24.4) | <0.001** | |
| NIHSS (11:18) | 2 (2.4) | 33 (42.3) | |||
| NIHSS (5:110) | 28 (33.7) | 23 (29.5) | |||
| NIHSS<5 | 53 (63.9) | 3 (3.8) | |||
| Stroke classification | LACS | 30 (36.1) | 10 (12.8) | <0.001** | |
| PACS | 29 (34.9) | 21 (26.9) | |||
| POCS | 23 (27.7) | 27 (34.6) | |||
| TACS | 1 (1.2) | 20 (25.6) | |||
| Hemodynamic and laboratory parameters on admission | SBP | 159.96 (15.97) | 154.53 (20.58) | 0.062 | |
| DBP | 101.87 (10.85) | 99.95 (12.65) | 0.302 | ||
| HR | 89.77 (13.05) | 97.23 (20.64) | 0.006** | ||
| RR | 21.31 (3.60) | 22.41 (5.14) | 0.117 | ||
| HB | 13.73 (1.51) | 13.58 (2.17) | 0.611 | ||
| TLC | 9.61 (2.06) | 14.49 (40.75) | 0.278 | ||
| CREAT | 8.66 (3.25) | 9.69 (4.18) | 0.082 | ||
| RBS | 188.49 (64.57) | 219.49 (105.28) | 0.025* | ||
| INR | 1.08 (0.15) | 1.52 (2.08) | 0.055 | ||
| SGPT | 27.77 (18.46) | 42.22 (38.10) | 0.002* | ||
| CHOL | 204.87 (38.36) | 222.42 (35.68) | 0.003* | ||
| TG | 151.57 (25.14) | 164.81 (35.02) | 0.006* | ||
| LDL | 101.60 (18.45) | 110.72 (23.32) | 0.006* | ||
| Transthoracic echocardiographic findings | Ejection fraction | EF>60% | 62 (74.7) | 27 (34.6) | <0.001** |
| EF 40-60% | 18 (21.6) | 45 (57.7) | |||
| EF<40% | 3 (3.6) | 6 (7.7) | |||
| Diastolic Dysfunction | No | 13 (15.7) | 1 (1.3) | <0.001** | |
| Grade 1 | 60 (72.3) | 50 (64.1) | |||
| Grade 2 | 10 (12.0) | 26 (33.3) | |||
| Grade 3 | 0 (0.0) | 1 (1.3) | |||
| LA dilatation | 19 (22.9) | 37 (47.4) | 0.002* | ||
| LVED | 2 (2.4) | 7 (9.0) | 0.142 | ||
| RSWMA | 5 (6.0) | 15 (19.2) | 0.021* | ||
| Mitral valve regurge | 3 (3.6) | 19 (24.4) | <0.001** | ||
Data expressed as mean ± SD or Number (percentage) as appropriate. *donates statistical significance p<0.05
**donates high statistical significance P<0.001
Abbreviations: CHF: Congestive Heart Failure; AF: Atrial Fibrillation; LACS: Lacunar Stroke; PACS: Partial Anterior Circulation Stroke Syndrome; POCS: Posterior Circulation Stroke; TACS: Total Anterior Circulation Stroke; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; HR: Heart Rate; RR: Respiratory Rate; Hb: Hemoglobin Level; TLC: Total Leukocytic Count; Creat: Serum Creatinine Level; RBS: Random Blood Sugar; INR: International Normalized Ratio; ALT: Serum Alanine Transaminase Level; CHOL: Serum Cholesterol Level; TG: Serum Triglyceride Level; LDL: Serum Low Density Lipoprotein Level; EF: Ejection Fraction; LA: Left Atrium; LVEDD: Left Ventricular End Diastolic Dilatation; RSWMA: Resting Systolic Wall Motion abnormalities
Table 5: Baseline demographics, clinical, laboratory, and transthoracic echocardiographic findings of good outcome group v/s poor outcome group.
Regarding ECG studies, our findings showed high statistically significant difference (p<0.001) in ECG between good and poor outcome group; poor outcome group had more incidence of tachycardia, QTc prolongation, T wave inversion, and ST segment changes (Figure 3).
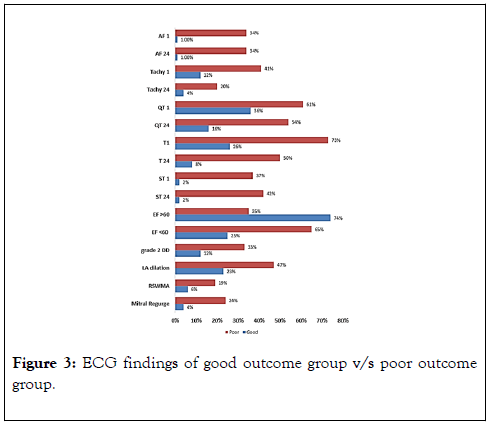
Figure 3. ECG findings of good outcome group v/s poor outcome group.
In terms of Echocardiography investigations, this study demonstrated that poor outcome group had higher resting systolic wall motion abnormalities when compared with good outcome group, with a statistical significant difference (p<0.05), and also, had a high statistical significant difference (p<0.001) regarding ejection fraction, diastolic dysfunction, LA dilation, and mitral valve regurgitation (Table 5).
Concerning the complications, Figure 4 showed that the poor outcome group had more incidence of NGT insertions, and need for MV and ICU compared with good outcome group (P<0.001). There was high statistically significant difference (p<0.001) in cTnT measurements between good and poor outcome groups regarding cTnT on admission, cTnT after 24 hours, and delta cTnT. Delta cTnT was 63 (80.8%) in poor outcome group and 27 (32.5%) in the good outcome group. The poor outcome group had higher cTnT on admission 32.15 (28.75%) and cTnT after 24 hours 49.90 (34.29%) when compared with the good outcome group 13.99 (7.53%) and 16.78 (10.83%), respectively.
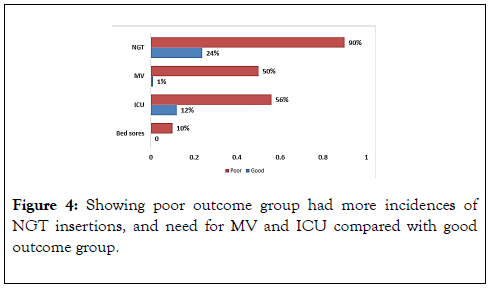
Figure 4. Showing poor outcome group had more incidences of NGT insertions, and need for MV and ICU compared with good outcome group.
Univariate and multivariate analysis
On Univariate analysis, NGT insertion, mechanical ventilation, intensive care unit admission, bed sores incidence, troponin on admission and after 24 hours, and dynamic troponin were significant predictors of poor outcome but only dynamic troponin remained a significant predictor of poor outcome in the multivariate regression model (OR 1.74, p=0.0174) (Tables 6 and 7). Table 8 and Figure 5 showed the receiver operating characteristic analysis.
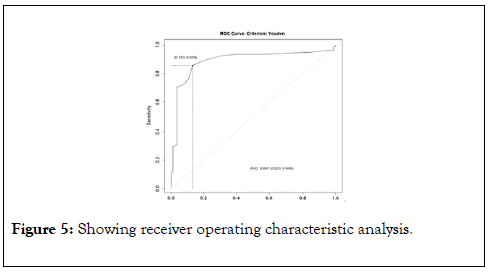
Figure 5. Showing receiver operating characteristic analysis.
| Univariate logistic | p value | Multivariate logistic | p value | |
|---|---|---|---|---|
| OR (95% CI for OR) | OR (95% CI for OR) | |||
| Comorbidities | ||||
| Age | 1.2 (0.9-1.4) | 0.00091 | 1.01 (1-1.03) | 0.178833 |
| CAD | 1.8 (1.3-2.6) | 0.0012 | 1.26 (0.7-2.26) | 0.439814 |
| CHF | 1.5 (0.87-2.6) | 0.15 | 0.7 (0.24-2.1) | 0.527411 |
| AF | 2 (1.3-3.1) | 0.0011 | 0.51 (0.18-1.39) | 0.186717 |
| ECG | ||||
| AF1 | 3.2 (2-5.3) | 0.0001 | 1.27 (1.18-2.23) | 0.22301 |
| AF24 | 3.8 (2.4-5.9) | 0.0001 | 1.27 (1.18-2.23) | 0.062449 |
| Tahy | 1.8 (1.3-2.6) | 0.00074 | 1.3 (0.67-2.54) | 0.441353 |
| Tachy24 | 2.1 (1.3-3.4) | 0.0026 | 0.74 (0.35-1.56) | 0.423354 |
| QT | 1.4 (1-1.9) | 0.025 | 0.74 (0.43-1.29) | 0.293591 |
| QT24 | 2 (1.4-2.7) | 0.0001 | 1.48 (0.76-2.91) | 0.252575 |
| T | 2 (1.4-2.7) | 0.0001 | 1.03 (0.61-1.74) | 0.919087 |
| T24 | 2.5 (1.8-3.6) | 0.0001 | 1.29 (0.63-2.63) | 0.485487 |
| ST | 3.6 (2.4-5.4) | 0.0001 | 0.7 (0.21-2.27) | 0.54952 |
| ST24 | 3.3 (2.2-5) | 0.0001 | 2.17 (0.64-7.38) | 0.215724 |
| BBB | 3.6 (1.4-8.8) | 0.0065 | 1.21 (0.31-4.77) | 0.786132 |
| Echocardiography | ||||
| EF>60 | 0.68 (0.3-1.2) | 0.0001 | 0.71 (0.15-3.44) | 0.673267 |
| DD2 | 1.3 (1.1-2.3) | 0.019 | 1.17 (0.7-1.96) | 0.550991 |
| LA dilation | 1.5 (1.1-2.1) | 0.016 | 0.91 (0.49-1.71) | 0.773538 |
| RSWMA | 1.7 (1-2.6) | 0.036 | 0.73 (0.33-1.61) | 0.434189 |
| Mitral regurge | 2.3 (1.4-3.6) | 0.0001 | 1.31 (0.63-2.73) | 0.474427 |
Abbreviations: OR: Odds Ratio, CAD: Coronary Artery Disease, CHF: congestive heart failure, AF: Atrial Fibrillation, Tachy: Tachycardia, QT: QT prolongation, T: T wave inversion, ST: ST segment changes, BBB: Bundle Branch Block, EF: Ejection Fraction, LA: Left Atrium, RSWMA: Resting Systolic Wall Motion Abnormalities.
Table 6: Univariate and multivariate logistic regression analysis of comorbidities, ECG and echocardiography for prediction of poor outcome.
| Univariate logistic | p value | Multivariate logistic | p value | |
|---|---|---|---|---|
| OR (95% CI for OR) | OR (95% CI for OR) | |||
| NGT | 2.7 (1.9-3.7) | 0.0001** | 1.5 (0.86-2.63) | 0.154279 |
| MV | 4.8 (3.2-7.4) | 0.0001** | 1.12 (0.52-2.42) | 0.775618 |
| ICU | 4.3(3-9.2) | 0.0001** | 1.1 (0.7-3.1) | 0.680774 |
| Sores | 3.7 (1.8-7.7) | 0.00055** | 0.61 (0.19-1.94) | 0.399807 |
| Troponin | 1.4 (1.3-1.9) | 0.0001** | 1.1 (0.98-1.3) | 0.138855 |
| Troponin24 | 1.8 (1.6-2.3) | 0.0001** | 1.2 (0.99-1.4) | 0.114879 |
| Troponin Dyn | 2 (1.4-2.7) | 0.0001** | 1.74 (1.1-2.74) | 0.017419* |
| NIH<18 | 5.16 (2.4-6) | 0.0001** | 4.17 (1.94-8.94) | 0.000252** |
| NIH<5 | 0.47 (0.43-0.92) | 0.74 (0.39-1.38) | 0.338932 | |
| NIH>18 | 6.3 (3.1-10) | 4.79 (1.64-13.95) | 0.004072* |
*donates statistical significance P<0.05
**donates high statistical significance P<0.001
OR: Odds Ratio, NIH: National Institute of Health, ICU: Intensive Care Unit, MV: Mechanical Ventilation, NGT: Nasogastric Tube Requirement
Table 7: Univariate and multivariate logistic regression analysis of complications, troponin measurements and NIHSS.
| AUC | 0.891 | ||
|---|---|---|---|
| Parameter | Estimate | 95% CI lower limit | 95% CI upper limit |
| Sensitivity | 0.8589744 | 0.76166101 | 0.927443 |
| Specificity | 0.8674699 | 0.77522826 | 0.931943 |
| PPV | 0.8589744 | 0.7624391 | 0.927443 |
| NPV | 0.8674699 | 0.77447967 | 0.931943 |
Abbreviations: AUC: Area Under the Curve, PPV: Positive Predictive Value, NPV: Negative Predictive Value
Table 8: ROC table for delta troponin.
The releasing of cardiac enzymes in acute stroke was first described by Norris et al. in 1979 [24] and James et al., [11] initially discussed the association between cTn and mortality in AIS. Later, several studies have examined the relation between cTns and stroke [25-27]. The etiology of increased levels of cTn in acute stroke is still incompletely understood. Jensen et al., [12] studied patients with AIS and with no overt coronary heart disease, his findings revealed that myocardial perfusion abnormalities were not significantly different in patients with elevated cTnT levels compared to patients with normal cTnT levels, suggesting that etiologies other than an acute coronary syndrome, such as congestive heart failure and renal failure, were responsible for the observed elevated troponin levels.
It has also been suggested that cardiac injury in relation to acute stroke is caused by patchy subendocardial hemorrhage or swollen myocytes surrounding epicardial nerves, a process known as myocytolysis [28]. Jespersen et al., proposed that increased catecholamine release, likely to originate from the insular cortex, could lead to an excessive release of intracellular calcium ions and thereby myocyte dysfunction [29]. Barber et al, found an association between elevated cTnI and epinephrine after ischemic stroke [10] Christensen et al, found an association between elevated cTnI and cortisol [30]. These findings may indicate a link between the sympathetic nervous system and myocardial cell damage in ischemic stroke patients.
In this study, hs-cTnT level was analyzed on admission and after 24 hours and revealed that 58.3% of AIS patients had elevated level of hs-cTnT on admission. This is in agreement with Scheitz et al. who found troponin elevation in 50% of patients [31]. While others found lower incidence; for example, Jensen et al. found 10% [12], James et al. found 17% [11], Barber et al. found 20% [10], Fure et al. found 9.6% [32] and Etgen et al. found 7.8% [15]. This high variability between this study and the previous studies could be explained by the use of the highly sensitive assay which allows detection of traces of troponin that is non-measurable by conventional assay. Also, the 99th percentile value was derived from healthy group which is noncomparable to AIS population. Lastly, we didn’t exclude patients with history of atrial fibrillation or Coronary artery disease or heart failure which have chronically elevated cTnT.
We analyzed hs-cTnT after 24 hours to differentiate between chronically elevated hs-cTnT and new onset elevation and thus creating two groups for analysis, the delta troponin group which we defined as 25% increase in the initial admission level, and the static group which did not meet that criterion. This study is the first to compare static and delta troponin regarding demographic data and patient characteristics. In this study, dynamic changes in troponin were found in 55.9% of our study population. While sheitz et al. found dynamic changes in 13.5% of their study population, this difference may be explained by their definition of dynamicity as increase more than 50% of the initial admission level.
Regarding demographic data and patient characteristics, our study revealed statistically significant difference between the static group and the delta troponin group who were older, had more prevalence of hypertension, had higher heart rate, and had significantly higher ECG ischemic changes on admission.
Electrocardiographic and echocardiographic findings in stroke
This study revealed a high prevalence of ECG abnormalities in the acute stage of ischemic stroke. The most frequent changes on ECG admission were AF (17.4%), Tachycardia (26%), prolonged QTc (48%), ST segment changes (21%), and T wave inversion (49%). This is in agreement with Fure et al., (32) who studied ECG changes in 279 strokes and described prolonged QTc (36.0%), ST depression (24.5%), atrial fibrillation (19.9%) and T wave inversion (17.8%). As pre-admission ECG records were lacking, so we could not differentiate between, pre and post stroke ECG changes. In order to solve this, we analyzed ECG changes on admission and again after 24 hours of and found statistical significance difference (p<0.05) in QTc prolongation 48% v/s 34%, tachycardia 42% v/s 11%, T wave inversion 49% v/s 28%, and ST segment changes 21% v/s 19%, respectively.
In this study, there was a statistically significant difference in ECG between static troponin group and delta troponin group regarding tachycardia; prolonged QTc, inverted T wave, and ST segment changes (p value<0.05) which were predictors of poor outcome in the univariate model. This is in agreement with Angelntonio et al., who found significant difference in stroke patients between positive troponin and negative troponin groups regarding AF, ST segment depression, and T wave inversion [14]. It has been suggested that prolonged QTc is associated with sudden death in some medical conditions, e.g. myocardial infarction, epilepsy [33], and subarachnoid hemorrhage [34].
Prognostic value of troponin
Our study defined poor outcome as MRS ≥ 3, and according to this definition, 48.4% had poor outcome within one month of the admission. There poor outcome group had high statistically significant difference regarding age, history of coronary artery disease and AF, severe stroke on admission (NIHSS>18), ECG changes, heart rate on admission, need for ICU admission, MV, bed sores, and NGT insertion. The poor outcome group had higher mean troponin value in admission and higher mean troponin value after 24 hours, higher incidence of dynamic changes in troponin values (25% increase in the initial value).
In order to find correlation between troponin elevation and poor outcome, we did univariate and multivariate logistic regression analysis. The univariate analysis as shown in Table 5 were all significant predictors of poor outcome but after adjusting the multivariate model, only the presence of AF after 24 hours in ECG proved to be independent factor of poor outcome, Table 6. These findings are in agreement with many previous studies in an observational study by James et al., of the 181 patients admitted for acute stroke, there was significant difference between good outcome and poor outcome groups regarding troponin T concentration (RR 3.2 [95% CI (1.7-5.8), p=0.0025). The study concluded that elevated troponin on admission in acute stroke patient is a strong predictor of mortality and carries a worse prognosis [11].
An analysis by 566 patients admitted for acute stroke showed that 212 of them had measured troponin I and had no clinical evidence of ACS (8%) had positive troponin in a retrospective trial by Raza et al. Patients were subdivided in positive troponin groups and normal troponin groups for 20.1 ± 10.3 months. This study found that high cardiac troponin is a strong predictor of long-term cardiac outcomes in patients with acute strokes [35]. Troponin T was increased in 20 (17.6%) patients in a separate prospective study by Abdi et al. that was conducted on 114 stroke patients. Troponin T in AIS patients is associated with increased age, creatinine, ECG, and severity of stroke; however, but location of stroke was not a determinant factor [36].
In a prospective study of 222 stroke patients that was conducted by Barber et al. they measured both cTnI and catecholamines, and they graded the ischemic damage on brain CT scan using the Alberta Stroke Program Early CT Score (ASPECTS). Researchers found that forty-five patients (20%) had troponin I>0.2 μg/l. These troponin-positive patients had higher epinephrine levels. They concluded that raised cTnI is associated with elevated circulating epinephrine. Nevertheless, increasing troponin is not associated with insular damage and does not predict poor AIS outcomes (10), independently. This is consistent with our findings, which show that static troponin is not an independent predictor.
In agreement with our study, Song et al. identified the relationships between elevated cTnT and stroke severity, location, and prognosis in the cohort of 416 patients. The elevated serum cTnT group showed many abnormalities on ECG and echocardiogram when compared to the normal serum cTnT group. Elevated cTnT was associated with severe neurological deficits at the onset of the stroke [37].
The acute Hs-cTnT elevation is high in acute ischemic stroke population, Hs-cTnT dynamicity within 24 hours is common, and delta troponin is correlated with stroke severity and poor outcome. Only delta troponin and poor NIHSS on admission proved to be independent predictors of short term (30 days) poor outcome.
None
None
None
Citation: Doha NM (2020) Highly Sensitive Cardiac Troponin T Changes and Its Prognostic Value in Patients with Acute Stroke. J Anesth Clin Res. 11:936. DOI: 10.35248/2155-6148.20.11.936.
Received: 28-Jan-2020 Accepted: 17-Feb-2020 Published: 24-Feb-2020 , DOI: 10.35248/2155-6148.20.11.936
Copyright: © 2020 Doha NM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.