
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Case Report - (2022)Volume 13, Issue 7
Congenital heart disease is one the most common birth defects. Survival improvement in recent decades caused an increasing number of children with congenital heart defects who undergo non-cardiac procedures with a mortality rate of 1% and an incidence of cardiovascular events of 11%. We present the case of a 6-month-old infant, with a history of GM1 gangliosidosis and dilated cardiomyopathy with left ventricular dysfunction, who underwent elective open surgery for a bowel biopsy and a gastrostomy. After preoperative risk stratification, general anesthesia was discarded in favor of a high-level spinal block under sedation. The procedure went uneventful and the baby maintained cardiovascular stability during the entire perioperative period. Spinal anesthesia may be a safe and effective option in infants with high-risk congenital heart defects. Proper local anesthetic selection and accurate dose calculation allow to perform upper abdominal procedures lasting up to 90 min.
Infant; Congenital heart defect; Cardiomyopathy; Spinal anesthesia
Congenital heart disease, one of the most common birth defects, is defined as a structural abnormality of the heart and/or great vessels that is present at birth. The overall incidence is 6-10 per 1000 live births and the adjusted mortality rate 4 per 100,000 population. Congenital heart defects associated with higher morbidity and mortality are single ventricle, aortic stenosis and cardiomyopathy [1-3].
Advances in diagnostic and therapeutic approaches have notably improved survival rates over the last decades, causing as well an increasing number of children with congenital heart defects, who undergo non-cardiac procedures under anesthesia and require more specific management. The mortality rate of these patients is 1% and the incidence of cardiovascular events is 11%. The main perioperative high-risk factors are the following: age <2 years, American Society of Anesthesiologists Physical Status (ASA-PS) ≥ 4, emergent or major surgery (intraperitoneal or intrathoracic procedures and those with large fluid shifts or blood losses), and major cardiac complications (cyanosis, pulmonary hypertension, heart failure, and arrhythmias) [1,4,5].
Written informed consent for the present publication was obtained from the parents.
We present the case of a 6-month-old term infant, ASA-PS IV and weighing 6 kg, who underwent elective open surgery for a small bowel biopsy and a gastrostomy through a supraumbilical midline vertical laparotomy. The patient had a history of GM1 gangliosidosis type I; dilated cardiomyopathy treated with digoxin, enalapril, furosemide, spironolactone, and carnitine; supravalvular pulmonary stenosis; facial dysmorphism; congenital cataracts; chronic malnutrition; glomerular proteinuria; abdominal distension (laparoscopic approach was contraindicated due to the risk of bowel injury) (Figure 1), and an acute bronchitis episode with hospital admission 2 weeks before surgery.
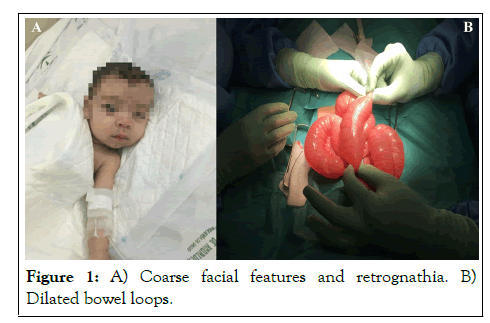
Figure 1: A) Coarse facial features and retrognathia. B) Dilated bowel loops.
On arrival to the anteroom, an anesthetic checklist was carried out and the lack of morning medication administration and a potential difficult airway due to coarse facial features and retrognathia were detected. Once in the operating room, fluid therapy with Isofundin® 50 ml/h, oxygen therapy through nasal prongs at 1.5 l/min, and dexmedetomidine 5 mcg bolus administration (over 5 min) were initiated. Spinal ultrasound was performed at L3-L4 interspace (distance from skin to subarachnoid space: 12 mm and from skin to anterior dura mater: 20 mm) prior to spinal block with hyperbaric 0.5% bupivacaine 7.5 mg (Figure 2).
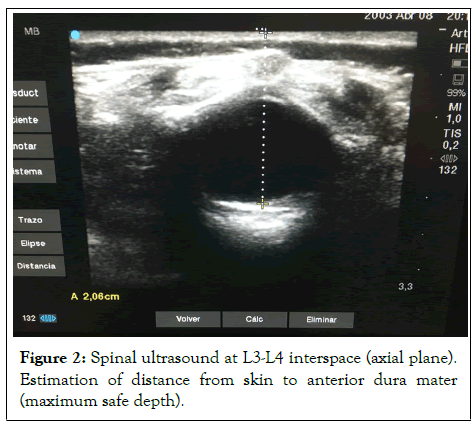
Figure 2: Spinal ultrasound at L3-L4 interspace (axial plane). Estimation of distance from skin to anterior dura mater (maximum safe depth).
From a technical point of view, special attention was paid to the local anesthetic injection time (>20s) to avoid a complete spinal block, given the marked abdominal distension and the use of a hyperbaric solution. Surgery commenced 8 min after the spinal block and confirmation of sensory level at T6 through a cold test with chlorhexidine (flinch or cardiorespiratory response). Likewise, cefazolin 180 mg+metamizole 200 mg+dexamethasone 3 mg+ranitidine 10 mg+magnesium sulphate 150 mg were administered. The following parameters were monitored: oxygen saturation, 98%-99%; heart rate, 120-140 bpm; mean arterial pressure, >50 mmHg; perfusion index, >0.8%; bispectral index, 60-70; temperature, >36.5ºC (Figure 3).
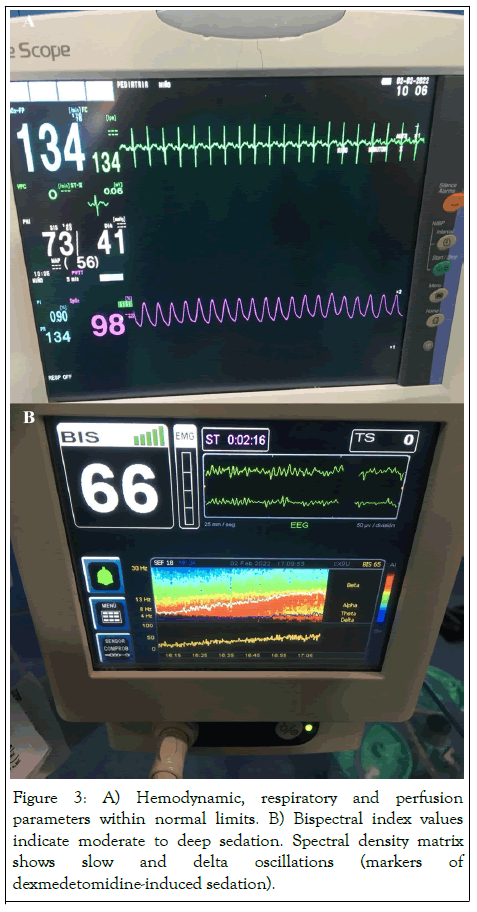
Figure 3: A) Hemodynamic, respiratory and perfusion parameters within normal limits. B) Bispectral index values indicate moderate to deep sedation. Spectral density matrix shows slow and delta oscillations (markers of dexmedetomidine-induced sedation).
The procedure went uneventfully and, once it was completed (duration of operative time was 57 minutes), the patient was moved to the Pediatric Intensive Care Unit (PICU). During the uneventful 6-hour stay in the PICU, the baby remained awake and stable with optimal hemodynamic, respiratory and perfusion parameters, as well as good scores on pain (Face, Leg, Activity, Cry, Consolability [FLACC] 0) and agitation (Pediatric Anesthesia Emergence Delirium [PAED] 0). The total duration of motor block was 80 min.
GM 1 gangliosidosis is an inherited (autosomal recessive) neurodegenerative disease characterized by a decreased beta-galactosidase activity that leads to lysosomal accumulation of GM1 gangliosides. The estimated incidence is 1 per 100,000-200,000 live births. It is classified into 3 subtypes according to the age of onset: type I or infantile (≤6 months), type II or juvenile (6 months-5 years) and type III or adult (5-18 years). Type I is the most common and severe, including psychomotor retardation, muscular hypotonia, vision loss, coarse facial features, distended abdomen, seizures, cardiomyopathy and resulting in death by 2 years of age due to heart failure or aspiration pneumonia [6].
Cardiomyopathy is defined as a disease of the myocardium associated with cardiac dysfunction, which can be present from birth. The incidence of cardiomyopathy in infants is 5 per 100,000. The main cardiomyopathy subtypes are dilated (60%), hypertrophic (25%), ventricular non-compaction (9%), and restrictive (2.5%). Dilated cardiomyopathy is mainly caused by myocarditis (46%) and neurometabolic disorders (26%), and is associated with a mortality rate up to 70%. Likewise, ventricular hypertrabeculation/non-compaction can be associated with neuromuscular diseases (up to 80%), and may have in addition to myocardial dysfunction-electrocardiographic changes, arrhythmias, ventricular dyssynchrony, and risk of thromboembolism (controversial) [7].
Anesthesia in children with dilated cardiomyopathy requires a preoperative cardiological evaluation, ideally consisting of the following: an ECG to detect rhythm, conduction or repolarization abnormalities; an echocardiogram to assess left ventricular function; a chest X-ray to assess the heart size, which could cause left mainstem bronchus extrinsic compression; and a review of cardiac medication, maintaining the morning administration and bearing in mind the potential anesthesia-induced hypotension associated with angiotensin-converting enzyme inhibitors (their perioperative maintenance or withdrawal is still controversial) or diuretic-induced hypokalemia (especially if digoxin therapy, due to risk of toxicity). Intraoperative management focuses on maintaining cardiac output and blood pressure, avoiding tachycardia and high systemic vascular resistance. General anesthetic agents causing myocardial depression should be avoided or reduced, especially in patients with severe ventricular dysfunction. The risk of adverse events increases in major or prolonged procedures, for which invasive hemodynamic monitoring and echocardiography should be considered; in case of heart failure, the use of inodilator agents (i.e., milrinone, dobutamine or levosimendan) or temporary cardiac pacing if ventricular dyssynchrony should be taken into account [1,7].
Pediatric spinal anesthesia is a safe and effective tool whose use is not widespread. However, it has the following advantages compared to general anesthesia (especially in neonates and ex-premature infants): lower incidence of hipoxemia, bradicardia and hypotension; less heat loss; lower incidence of postoperative early apnea (first 30 min); and reduced anesthesia times, time to operating room exit and time to first feed. Therefore, high-risk infants (i.e., ex-prematures and/or those with cardiorespiratory disease) are target patients for spinal block. The main limitations when using this technique are the motor block duration (<90 min) and sensory block level (≤ T10), which reduce the spectrum of surgical indications mostly to infraumbilical procedures lasting less than 90 min (in practice, it is difficult to achieve a T10 sensory block for over 60 min). In our hospital, the duration and sensory level are optimized by the following two steps: 1) local anesthetic solution is selected based on estimated surgical time (taking advantage of the pharmacological properties of the local anesthetics): hyperbaric 0.5% bupivacaine when estimated surgical time is <60 min, isobaric 0.5% bupivacaine when it is 60-75 min, and isobaric 0.5% bupivacaine with epinephrine (1:200,000) when it is 75-90 min; 2) doses are calculated using a self-developed dosing chart based on age and sensory level (Table 1) [8-10].
| Eizaga chart | |||
|---|---|---|---|
| Age (mo) |
<T10 block (mg/kg) |
≥ T10 block (mg/kg) |
Maximum doses (mg) |
| <6 | 1 | 1.25 | 5 |
| 06-12 | 0.75 | 1 | 7.5 |
| >12 | 0.5 | 0.75 | 10 |
Table 1: Pediatric spinal anesthesia dosing.
Regarding our case, the baby was diagnosed with GM1 gangliosidosis after dilated cardiomyopathy was detected during a hospital admission due to congestive heart failure. Preoperative cardiological evaluation included a normal ECG; a chest X-ray, which showed cardiomegaly, mediastinal widening and pulmonary vascular congestion; and an echocardiogram, which detected left ventricular dilatation and hypertrabeculation (without non-compaction criteria), septal dyskinesia, systolic dysfunction, and supravalvular pulmonary stenosis. Preoperative risk assessment determined that the baby was at high risk for cardiovascular events due to age <2 years, ASA-PS IV, intraperitoneal surgery, and heart failure; and at increased risk for respiratory events, due to the potential difficult airway (coarse facial features and retrognathia) and the recent episode of acute bronchitis. All in all, we decided to avoid general anesthesia and to perform a high-level spinal block under sedation. From a technical point of view, hyperbaric 0.5% bupivacaine was used as the estimated surgical time was <60 min and a dose of 1.25 mg/kg was calculated as the targeted sensory level was T6 (supraumbilical midline laparotomy). Procedural sedation was performed with dexmedetomidine (0.8 mcg/kg) for two reasons: first, to counteract potential tachycardia and high systemic vascular resistance given its sympatholytic effect, and bearing in mind that the morning cardiac medication had not been administered; secondly, to maintain a patent airway and spontaneous ventilation, without manipulating the potential difficult airway. Finally, the baby maintained cardiorespiratory stability, comfort and analgesia throughout the entire perioperative period.
Pediatric spinal block may be a safe and effective option that allows to avoid general anesthesia in infants with high-risk congenital heart defects. The upper abdominal surgery can be considered fairly safe and specially recommended for conducting upper abdominal surgeries in developing countries where cost factor is important. Further controlled studies will be required to establish the safety of high SA for different types of upper abdominal surgeries and to bring out the appropriate protocols and guidelines. Proper local anesthetic selection and accurate doses calculation according to the estimated surgical time and sensory level, respectively, allow performing upper abdominal procedures lasting up to 90 min.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Rebollar RE, Tapia BG (2022) High-Level Spinal Block for Upper Abdominal Surgery in an Infant with Cardiomyopathy. J Anesth Clin Res. 13:1072.
Received: 03-Jun-2022, Manuscript No. JACR-22-18841 ; Editor assigned: 06-Jun-2022, Pre QC No. JACR-22-18841 (PQ); Reviewed: 22-Jun-2022, QC No. JACR-22-18841 ; Revised: 28-Jun-2022, Manuscript No. JACR-22-18841 (R); Published: 05-Jul-2022 , DOI: 10.35248/2155-6148.22.13.1072
Copyright: © 2022 Rebollar RE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.