Journal of Hematology & Thromboembolic Diseases
Open Access
ISSN: 2329-8790
ISSN: 2329-8790
Research - (2023)Volume 11, Issue 6
Objective: To evaluate the accuracy of the Hematologic Scoring System as a tool for the early diagnosis of neonatal sepsis
Methods: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram was utilized for identification, screening and identification of eligible studies. Databases (PubMed, Herdin, Cochrane Library, Medline, Ovid, Google Scholar) were searched for relevant studies involving Hematologic Scoring System (HSS) by included studies were assessed for quality and risk of bias. Study design such as cross-sectional, randomized controlled trials, case controls that looked into neonates, term (>37weeks) or preterm (<37weeks), admitted in the Neonatal Intensive Care unit, with age from birth for up to 28 days of life, with clinical suspicion of neonatal sepsis were included.
Results: The Hematologic Scoring System (HSS) score of 3 and above as cut-off is accepted for initiation of treatment. The study showed a pooled diagnostic odds ratio of 34.55 (95% CI: 14.22-83.93). The pooled sensitivity is at 0.41 (0.38-0.44) at 95% CI and a specificity of 0.99 (0.98-1.00) at 95% CI. However, there is high statistical heterogeneity
Conclusion: This systematic review and meta-analysis analyzed fair to good quality evidences which revealed that the Hematologic Scoring System (HSS) is an accurate and a reliable screening tool for the diagnosis of Neonatal Sepsis. It is a quick and economical screening tool that is objective and thus increases the weight of a regular Complete Blood Count (CBC). Mostly, the source of studies that were used in this meta-analysis usually lacks specific information on the background of the neonates; prenatal and postnatal, the time when the extraction was done and other issues surrounding a sick neonate. A more rigorous and meticulous study design such as a randomized controlled trials made in our setting are needed to further determine its accuracy in diagnosing neonatal sepsis.
Hematologic scoring system; Neonatal sepsis; Diagnostic tools
Neonatal sepsis is an infection in the bloodstream in newborn infants less than 28 days old. It remains to be a leading cause of morbidity and mortality among infants especially in the middle and low-income countries [1]. Neonates are at the highest risk for bacterial sepsis at a prevalence of 1 to 10 per 1000 live births worldwide [2]. In the Philippines in 2015, approximately there were 2,300,000 babies born at around 6,400 every day. The Neonatal Mortality Rate (NMR) was 13 deaths per 1,000 live births. NMR in rural areas was 18 deaths per 1,000 live births and 9 deaths per 1,000 live births in urban areas [3]. Around 13% of newborn deaths is caused by neonatal sepsis with prematurity as the leading factor at 32.7% [3].
Neonatal sepsis is caused by both bacterial and non-bacterial organisms such as viruses, fungi, protozoans and mycoplasma species. These organism are commonly present in the genitourinary and lower gastrointestinal tracts of mothers. The most common causative bacterial organisms are Escherichia coli and group B Streptococcus [4]. The fetus can become infected during the intrapartal or postpartal stages. Clinical manifestations of neonatal sepsis are most of the time non-specific and involve multiple organ systems. Clinical signs include temperature instability, hypotension, poor perfusion manifesting as pallor or mottled skin, metabolic acidosis, tachycardia or bradycardia, apnea, respiratory distress, grunting, cyanosis, irritability, lethargy, seizures, feeding intolerance, abdominal distention, jaundice, petechiae, purpura, and bleeding. Initial symptoms might be few, and include apnea alone or tachypnea with retractions, nasal flaring, grunting, or tachycardia [5]. Sepsis may be classified according to onset: Early onset and late onset. Seven days or 1 week is the arbitrary cut off to say if it’s early or late onset. Other studies use a different definition. Early onset is considered if infection is apparent in less than 24 hours to 3 days while late-onset if signs appear after 3 days up to 60 days [6]. Early onset infections are acquired before or during delivery (vertical mother-to-child transmission). Late onset infections develop from organisms acquired in the hospital or at the community.
Blood culture and sensitivity studies remains as the gold standard in diagnosing neonatal sepsis. The procedure requires several materials such as sterile gloves, povidone iodine solution or chlorhexidine solution, culture bottles, agar plates and incubation machines. The turnaround time for a laboratory to release results for Gram stain, organism identification, and antimicrobial sensitivity is approximately 1, 2, and 3 days, respectively [7].
Complete blood count with differential count is routinely requested as an adjunct diagnostic test in diagnosing neonatal sepsis. It evaluates the Red Blood Cells (RBC), platelets, White Blood Cells (WBC) and its differential count. Its turnaround time is relatively fast, and may only require basic laboratory materials such as a microscope. The platelet count of a healthy newborn is rarely lower than 100,000/μL in the first 10 days of life (normal, ≥150,000/ μL). Thrombocytopenia (platelet count<100,000/µL) may be a presenting sign of neonatal sepsis and can last as long as 3 weeks. About 10%-60% of infants with sepsis have thrombocytopenia [8]. A study done in 2017 looked into the association of thrombocytopenia with neonatal sepsis, they found that of the 460 who were confirmed for sepsis out of 6551 neonates, 92 developed thrombocytopenia at a sensitivity of 20%. With a specificity of 97.8%, a multivariate analysis was done and showed, maternal hypertension, intravascular thrombosis and gram negative sepsis were independently associated with thrombocytopenia in neonatal sepsis [9].
WBC count is a more sensitive indicator for sepsis than platelets. However, it is nonspecific and has a low positive predictive value. Normal WBC counts may be initially observed in as many as 50% of cases of culture-proven sepsis. Infants who are not infected may also demonstrate abnormal WBC counts related to the stress of delivery and other factors. A low WBC count (<5,000/µL) is associated with a higher likelihood ratio for sepsis than an elevated WBC count (>20,000/µL) [9].
A study done by Hornik, et al. [10] determined the association of CBC count indices, namely WBC count, Absolute Neutrophil Count (ANC), Immature-to-Total neutrophil (I/T) ratio, and platelet count, with culture results of neonates admitted to 293 NICUs in the United States suspected early onset sepsis. They found that specificity and negative predictive values were high-73.7 to 99.9% and >99.8%, respectively. Sensitivity was low (0.3%- 54.5%) for all complete blood cell count indices analyzed. They concluded that low WBC count, ANC, and high I/T ratio were associated with increasing odds of infection. However, none of the CBC-derived indices could reliably rule out early onset neonatal sepsis.
Another study looked into the association of CBC indices with neonates who were presenting signs of late onset sepsis. It found that high and low WBC counts, high ANC, high I/T ratio, and low platelets were associated with late onset sepsis. Specificity was highest for white blood cell counts of <1000/mm3 or >50,000/ mm3 (>99%). Positive likelihood ratios were highest for white blood cell counts<1000/mm3 (4.1) and platelet counts <50,000/ mm3 (3.5) [11].
With current developments on neonatal sepsis, the condition remains hard to diagnose when blood culture is negative [12]. Clinicians rely on signs and symptoms when deciding to initiate antibiotic therapy. In a study done by de Man, et al. [12] unnecessary antibiotic treatment in the neonatal period disturbed the microbial flora colonizing the neonate and may lead to colonization with multi-drug resistant bacterial strains [13].
Rodwell, et al. [13] developed a screening tool utilizing the CBC indices called the Hematologic Scoring System. Scores were assigned for each of the 7 findings: Abnormal total leukocyte count, Abnormal total neutrophil (PMN) count, elevated immature PMN count, elevated immature to total PMN ratio, immature to mature PMN ratio >0.3, platelet count less than or equal to 150,000/mm3, and pronounced degenerative changes in PMNs. In the study, out of the 298 evaluations for sepsis, 27 were confirmed with an actual sepsis and 26 of which at 96% had scores of more than or equal to 3 with a higher likelihood of sepsis whereas those who got 2 and below had lower likelihood for sepsis [14].
There were a number of studies to evaluate the HSS. Majumdar, et al. [14] utilized the HSS in 60 neonates admitted in the Neonatal Intensive Care Unit (NICU). They assessed its performance for the 20 proven septic cases and concluded that HSS increases the diagnostic accuracy of the complete blood cell count as a screening test for sepsis while simplifying and standardizing its interpretation. Another study by Makkar, et al. [15] had 110 neonates who were clinically suspected to have bacterial infection within 1st week of life, based on perinatal risk factors and clinical features. Results revealed 83.33% of infants with culture proven sepsis had HSS scores suggesting sepsis. However 21.74% of healthy infants had similar scores16. Out of 42 cases with culture proven sepsis, 35 (83.33%) infants had score ≥ 5, and 7 (16.67%) had scores 3-4. 12 (54.55%) cases with probable infection had scores 3-4; 6 (27.27%) had score ≥5. 10 (21.74%) of the normal infants had score ≥ 5 suggesting the presence of sepsis and 13 (28.26%) had scores 3-4 suggesting the possibility of sepsis in these cases, thus started with antibiotic treatment. 23 (50%) of the normal infants and 4 (18.18%) with probable infection had score ≤ 2 which implies that sepsis was unlikely in these cases.
The feasibility and the cost effectiveness of the system increase its usefulness. This helps the clinicians to reach a probable diagnosis, decreasing the death toll and institute a rational approach towards the patient medication, i.e., avoiding unnecessary introduction of antibiotics and preventing the development of resistance to drugs. There are no/very few studies of this system in our country.
Relevance of the study
Neonatal sepsis remains to be a leading cause of morbidity and mortality among infants. Neonates are at the highest risk for bacterial sepsis. Although a good clinical history and a complete physical examination are important in considering sepsis, a reliable and objective screening tool is helpful especially in situations when blood culture cannot be done, or when results are still unavailable.
Currently, a basic complete blood count with platelet count is primarily requested when considering neonatal sepsis. More tests are sometimes requested such as c-reactive protein and procalcitonin. Such tests requires laboratory set ups, machines, and skilled technicians which primary and most secondary to even tertiary healthcare facilities lack. These tests are also costly to most Filipinos.
This study intends to review existing knowledge on the accuracy of the Hematologic Scoring System (HSS) by Rodwell, et al. [13] in screening for neonatal sepsis. Results of this meta-analysis will serve as a collaborative and evidence-based rationale for its utilization in screening and diagnosing neonatal sepsis.
Objectives
General objective: To evaluate the accuracy of the Hematologic Scoring System as a tool for the early diagnosis of neonatal sepsis
Specific objectives: To determine the basic prenatal background of neonates presenting with clinical signs of sepsis
1. To determine the most common presenting signs of neonatal sepsis
2. To determine the sensitivity, specificity, negative likelihood ratio and positive likelihood ratio of the Hematologic Screening Tool by Rodwell, et al. [13].
3. To determine difference in likelihood of the HSS considering when the extraction was done (early-onset and late onset sepsis)
Search strategy
The two investigators primarily searched electronic databases (PubMed, Cochrane, Herdin, Medline, Google scholar) dated January 2010 until the last week of August 2020. Medical Subject Heading (MeSH) terms and free text terms were used in the search which included “Hematologic Scoring System” or “HSS” and “neonatal sepsis” and “sepsis neonatorum” and “diagnostic”. The search was not limited to the aforementioned electronic databases, a manual search for both published and unpublished researches and their references was done and was both screened and included if were deemed appropriate.
Eligibility criteria
Studies were selected independently by the two investigators. Irrelevant studies based on abstracts and titles were excluded and the remainder were further evaluated for eligibility. Study design such as cross-sectional, randomized controlled trials, case controls that looked into neonates, term (>37 weeks) or preterm (<37 weeks), admitted in the Neonatal Intensive Care unit, with age from birth for up to 28 days of life, with clinical suspicion of neonatal sepsis were included . Studies included utilized the Hematologic Scoring System developed by Rodwell, et al. [13] based on a Complete Blood Count (CBC) with Differential counts. Abstracts only, studies for pay, case reports, narrative reviews, animal studies, studies not written without English translation and in vitro studies were not included.
Study selection and quality assessment and risk for bias tools
The two investigators utilized the National Institutes of Health (NIH) quality assessment tool for observational cohort and crosssectional study and the Cochrane Collaboration Tool. Screening and determination of eligibility were done independently by the 2 assessors. Any issues on bias were raised in the middle of the study and sought the aid of the research adviser in settling disputes to reach a consensus. The investigators adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta- Analyses) flow for identification, screening and identification of eligible and included studies.
Data collection and items
Information collected were not limited to the actual figures in the included studies. Information on the first author, year of publication, country, study design were also recorded. Results of HSS scoring, clinical diagnosis and actual result of the blood culture studies were also included. Information on the perinatal condition of the pregnancy if available were taken together with gender, and the subject’s presenting signs and symptoms. The two investigators extracted data such as age of gestation, gender, weight for pediatric age (appropriate for gestational age, small for gestational age, large for gestational age), manner of delivery and other possible aggravating prenatal events. The hour of life (mean+SD) at which the complete blood count was taken were also noted (mean+SD). A separate analysis was to be released for each item to show possible difference in sensitivity and specificity, however only one study presented data that is incomplete, hence is not useful.
Analytics synthesis of results
Statistical analysis for the diagnostic threshold was performed using the Meta-disc v1.4. Since this study reviews outcome for diagnostic significance, data outcomes were analyzed as Diagnostic Odds Ratio (DOR) to assess the accuracy of the Hematologic Scoring System in the diagnosis of Neonatal Sepsis. A random-effect model was to calculate the pooled DOR. The I2 was used to quantify the degree of heterogeneity. An I2 >50% indicated high heterogeneity. An unrestricted maximum likelihood random-effects meta-regressions will be performed.
Literature and search selection
A total of 22,229 studies were identified from the initial online search from electronic databases. After removing duplicates, 22,211 studies remained for title and abstract screening. A total of 124 studies were retrieved for full text review. 18 studies met the eligibility criteria and were included. The final screening on qualitative analysis left 12 studies for inclusion consisting of 10 prospective analytic studies and 2 cross-sectional studies. Bulk of the search results using Google scholar yielded like studies from other search engines. Also, it included studies that utilize other hematologic scoring systems other that Rodwell, et al. [13] and for oncology cases. Figure 1 shows the PRISMA flow chart to illustrate the study selection process.
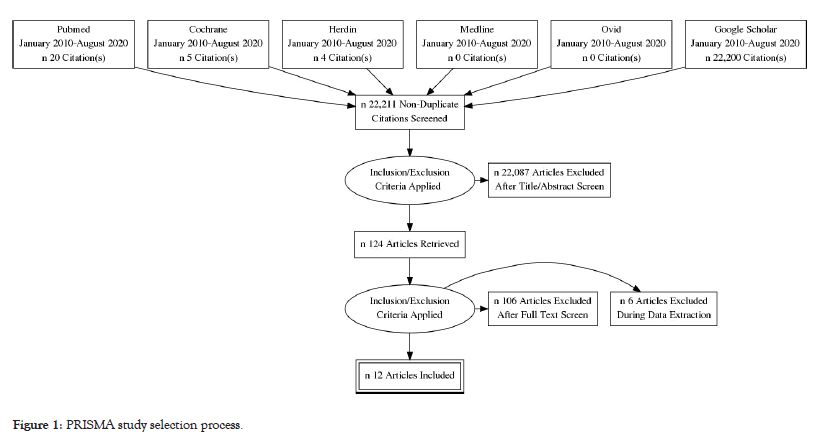
Figure 1:PRISMA study selection process.
Study characteristics
There were 12 studies were selected for inclusion with a total of 1,481 participants. Countries of origin was predominantly from India (10) with one from Pakistan and the other from Bangladesh.
Extracted and recalculated data from the studies include, sepsis status with HSS scores (>3 or >5), diagnostic profile, and odds ratio for effect size. Tables 1 and 2 shows the characteristics of included studies.
| Author | Study type | Year | Country | Total sample (n) | Gender (n) | Intervention | Comparator | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ||||||||
| Ahirrao, et. al [16] | Prospective-Ananalytic | 2016 | India | 303 | 188 | 115 | HSS(CBC), CRP | Blood Culture | Role of HSS in early diagnosis of neonatal sepsis |
| Ali, et al. [17] | Prospective-Ananalytic | 2019 | Pakistan | 100 | 62 | 38 | HSS(CBC), CRP | Blood Culture | Role of HSS in early diagnosis of neonatal sepsis |
| Chaudhari, et a.l [18] | Prospective-Ananalytic | 2017 | India | 60 | 48 | 12 | HSS(CBC), CRP | Blood Culture | Role of HSS in early diagnosis of neonatal sepsis, to know whether the value for a particular parameter and the difference between EONS and LONS was significant or not. |
| Chaware, et al. [19] | Prospective -Ananalytic |
2016 | India | 160 | 100 | 60 | HSS(CBC) | Blood Culture | Evaluate and highlight the importance of simple, quick, cost effective hematological scoring system which may aid clinicians to reach a probable diagnosis, avoiding unnecessary use of antibiotics and decreasing death rate |
| Debroy, et al. [20] | Prospective -Ananalytic | 2016 | India | 40 | 17 | 23 | HSS(CBC) | Blood Culture | Evaluate the diagnostic accuracy of total HSS and its individual seven components, for diagnosis of neonatal sepsis as evidenced by blood culture (definite neonatal sepsis) |
| Dutta, et al. [21] | Cross-secti onal | 2016 | India | 210 | 119 | 91 | HSS(CBC), CRP | Blood Culture | Evaluating the Haematological Scoring System (HSS) in early diagnosis of neonatal sepsis and to find out its significance |
| Lal, et al. [22] | Prospective -Ananalytic | 2019 | India | 110 | 66 | 44 | HSS(CBC), CRP | Blood Culture | Evaluate the utility of the (HSS) in the early diagnosis of neonatal sepsis |
| Sawadkar, et al. [23] | Prospective-Ananalytic | 2020 | India | 100 | 46 | 54 | HSS(CBC) | Blood Culture | Evaluate the HSS for early diagnosis of neonatal sepsis and to find out its significance. |
| Narasimha, et a.l [24] | Prospective-Ananalytic | 2009 | India | 50 | 25 | 25 | HSS(CBC) | Blood Culture | Assess the significance of the Hematological Scoring System (HSS) for early detection of neonatal sepsis |
| Alva, et al. [25] | Prospective -Ananalytic | 2015 | India | 110 | 72 | 38 | HSS(CBC), CRP | Blood Culture | Assess the signififi- cance of the Hematological Scoring System (HSS) for early detection of neonatal sepsis |
| Yusuf, et al. [26] | Prospective -Ananalytic | 2014 | Bangladesh | 128 | 84 | 44 | HSS(CBC), CRP | Blood Culture | To evaluate the haematological scoring system for early diagnosis of neonatal sepsis and to find out its significance. |
| Makkar, et al. [15] | Cross-secti onal | 2013 | India | 110 | 54 | 56 | HSS(CBC) | Blood Culture | To evaluate and highlight the importance of HSS in the early detection of neonatal sepsis. |
Table 1: Characteristics of included studies.
| Author | Year | Participants n | HSS score | Blood Culture status | OR (CI:95%) | p-value | |
|---|---|---|---|---|---|---|---|
| Sepsis n | No sepsis n | ||||||
| Ahirrao, et al. [16] | 2016 | 303 | >3 | 77 | 168 | 53.8(3.3-882.0) | 0.0052 |
| <3 | 0 | 58 | |||||
| Ali, et al. [17] | 2019 | 100 | >3 | 33 | 53 | 18.2(1.1-314.6) | 0.05 |
| <3 | 0 | 14 | |||||
| Chaudhari, et al [18] | 2017 | 60 | >3 | 46 | 0 | 2697(51.2-142056.2) | 0.0001 |
| <3 | 0 | 14 | |||||
| Chaware, et al. [29] | 2016 | 160 | >3 | 28 | 48 | 99.3(5.9-1663.2) | 0.0014 |
| <3 | 0 | 84 | |||||
| Debroy, et al [20] | 2016 | 40 | >3 | 9 | 13 | 11.7962(1.319-105.0125) | 0.0272 |
| <3 | 1 | 17 | |||||
| Lal, et al [22] | 2019 | 110 | >3 | 46 | 30 | 105.2(6.2-1780.8) | 0.0013 |
| <3 | 0 | 34 | |||||
| Sawadkar, et al. [23] | 2020 | 100 | >3 | 29 | 41 | 18(1.0-325.5) | 0.051 |
| <3 | 0 | 30 | |||||
| Narasimha, et al. [24] | 2009 | 50 | >3 | 12 | 26 | 11.8(0.6-215.5) | 0.096 |
| <3 | 0 | 12 | |||||
| Alva, et al. [25] | 2015 | 110 | >3 | 23 | 37 | 63.3(3.7-1075.6) | 0.0041 |
Table 2: Characteristics of included studies: HSS versus blood culture result.
Risk of bias in included studies
The two investigators agreed in the assessment of the risk for bias of the included studies based on the NIH Quality Assessment Tool for Observational Cohort and Cross-sectional Studies. The included studies have addressed appropriate source population, measurement methods, study design and statistical method. All included studies had a rating of good which also means least amount of bias.
Outcome evaluation and meta-analysis
The analysis of diagnostic threshold was done using Meta-DiSc v1.4. Diagnostic Odds Ratio (DOR) was computed as the measure of performance. The HSS presents two scoring category where sepsis is probable or more likely and would suggest treatment, computation of the needed values was done for the >3 cut off (Spearman correlation coefficient: 0.063 p-value=0.846) and the >5 cut off: (Spearman correlation coefficient: 0.255 p-value 0.450) such as the Diagnostic Odds Ratio, sensitivity, specificity, positive likelihood ratio and negative likelihood ratio. Using the score of 3 and above as cut-off, using the random effects model, the pooled diagnostic odds ratio was at 34.55 at 95% CI (14.22-83.93). The I-square is 29.8% and the Cochran Q is 15.67%. Here, there is low statistical significant heterogeneity across studies (Figure 2).
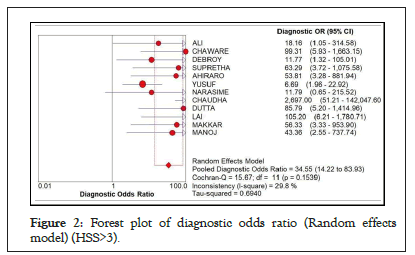
Figure 2: Forest plot of diagnostic odds ratio (Random effects model) (HSS>3).
Still using the HSS cut-off of >3, in Figure 3, the result showed a high pooled sensitivity of 0.41 at 95% CI. However, the Chi-square was at 115.32 and I-square of 90.5% signifying high heterogeneity across studies.
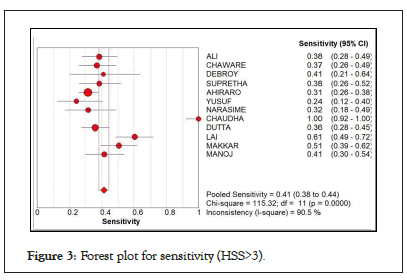
Figure 3: Forest plot for sensitivity (HSS>3).
As seen in Figure 4, there is a high pooled specificity value at 0.99 (95% CI=0.98-1.00). Almost all of the results lie close and within the scope of the true value. The chi-square value was low at 15.91 and the I-square was only at 30.8%, low heterogeneity.
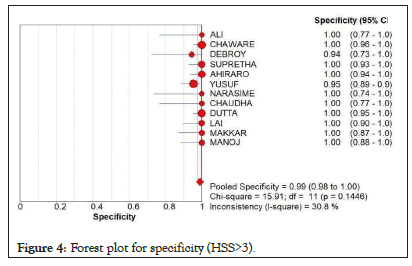
Figure 4: Forest plot for specificity (HSS>3).
In Tables 3 and 4 shows computed likelihood ratio. In Table 3, the summary of positive likelihood ratio, the pooled LR+ is high at 13.944 (95% CI: 7.313-26.590). Significant statistical heterogeneity was low at 10.33 with p value of 0.501. In table 4, the LR- using the cut-off score of 3 is only at 0.6 (95% CI: 0.556-0.690).
| Study | | LR+ | [95% Conf. Interval.] | % Weight | |
|---|---|---|---|---|
| Ali, et al. [17] | | 11.552 | 0.747 | -178.57 | 5.56 |
| Chaware, et al. [29] | | 62.922 | 3.908 | -1013.2 | 5.39 |
| Debroy, et al. [20] | | 7.364 | 1.027 | -52.794 | 10.74 |
| Alva,et al. [25] | | 39.295 | 2.447 | -631.09 | 5.4 |
| Ahirrao, et al. [16] | | 37.175 | 2.338 | -591 | 5.44 |
| Yusuf et al. [26] | | 5.305 | 1.769 | -15.913 | 34.53 |
| Narasimha, et al. [24] | | 8.333 | 0.529 | -131.16 | 5.48 |
| Chaudhari, et al. [18] | | 29.681 | 1.945 | -453 | 5.61 |
| Dutta, et al. [21] | | 55.326 | 3.46 | -884.67 | 5.42 |
| Lal, et al. [22] | | 42.273 | 2.681 | -666.49 | 5.48 |
| Makkar, et al. [15] | | 28.333 | 1.802 | -445.46 | 5.49 |
| Sawadkar, et al. [23] | | 25.761 | 1.625 | -408.31 | 5.46 |
| (REM) pooled LR+ | | 13.944 | 7.313 | -26.59 | - |
| Note: Heterogeneity chi-squared=39.27 (d.f.=11) p=0.000; Inconsistency (I-square)=72.0 %; Estimate of between-study variance (Tau-squared)=0.0226 No. studies=12; Filter off add 1/2 to all cells of the studies with zero. | ||||
Table 3: Summary negative likelihood ratio (Random effects model)(HSS>3).
| Study | | LR+ | [95% Conf. Iterval.] | % Weight | |
|---|---|---|---|---|
| Ali, et al. [17] | | 11.552 | 0.747 | -178.57 | 5.56 |
| Chaware, et al. [19] | | 62.922 | 3.908 | -1013.2 | 5.39 |
| Debroy, et al. [20] | | 7.364 | 1.027 | -52.794 | 10.74 |
| Alva, et al. [25] | | 39.295 | 2.447 | -631.09 | 5.4 |
| Ahirrao, et al. [16] | | 37.175 | 2.338 | -591 | 5.44 |
| Yusuf et al. [26] | | 5.305 | 1.769 | -15.913 | 34.53 |
| Narasimha, et al. [24] | | 8.333 | 0.529 | -131.16 | 5.48 |
| Chaudhari, et al. [18] | | 29.681 | 1.945 | -453 | 5.61 |
| Dutta, et al. [21] | | 55.326 | 3.46 | -884.67 | 5.42 |
| Lal, et al. [22] | | 42.273 | 2.681 | -666.49 | 5.48 |
| Makkar, et al [15] | | 28.333 | 1.802 | -445.46 | 5.49 |
| Sawadkar, et al [23] | | 25.761 | 1.625 | -408.31 | 5.46 |
| (REM) pooled LR+ | | 13.944 | 7.313 | -26.59 | - |
| Note: Heterogeneity chi-squared=10.33 (d.f=11) p=0.501; Inconsistency (I-square)= 0.0 %; Estimate of between-study variance (Tau-squared)=0.0000 No. studies=12; Filter off add 1/2 to all cells of the studies with zero | ||||
Table 4: Summary positive likelihood ratio (Random effects model) (HSS>5).
Particularly looking at the analysis when the cut-off score would be raised to 5 and up, Figure 5 shows the pooled diagnostics odds ratio at 32.29 (95% CI: 14.31-72.88), a Chocran Q value of 32.85 and an I square of 69.6% suggesting a high heterogeneity. However, it also shows that results lie within the 95% CI except for the studies of Yusuf, et al. [26].
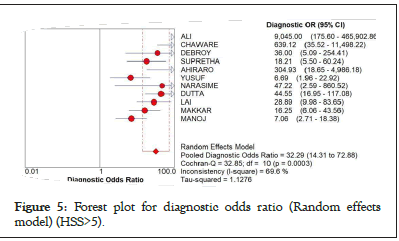
Figure 5: Forest plot for diagnostic odds ratio (Random effects model) (HSS>5).
At the HSS cut-off of 5 and up, Figure 6 showed a pooled sensitivity of 0.60 (at 95% CI: 0.56-0.65), chi-square of 94.53 and I-square of 89.4% with lesser sensitivity to sepsis and significant statistical heterogeneity.
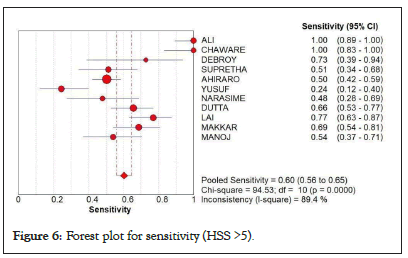
Figure 6: Forest plot for sensitivity (HSS >5).
In Figure 7, showing the forest plot on the specificity when HSS cut off was raised to 5 and up. Results showed a pooled specificity of 0.95 (95% CI: 0.93-0.96) and I-square of 75.2%. High heterogeneity was noted but with noted high score for specificity.
In Tables 5 and 6, shows computed likelihood ratio. In Table 5, the summary of positive likelihood ratio, the pooled LR+ is high at 13.944 (95% CI: 7.313-26.590). Significant statistical heterogeneity was low at 10.33 with p value of 0.501. In table 4, the LR- using the cut-off score of 3 is only at 0.619 (95% CI: 0.556-0.690) (Figure 8).
| Study | | LR- | [95% Conf. Iterval.] | % Weight | |
|---|---|---|---|---|
| Ali, et al. [17] | | 0.636 | 0.526 | -0.77 | 9.42 |
| Chaware, et al. [19] | | 0.634 | 0.533 | -0.753 | 9.98 |
| Debroy, et al. [20] | | 0.626 | 0.434 | -0.902 | 5.28 |
| Alva, et al. [25] | | 0.621 | 0.508 | -0.759 | 9.15 |
| Ahirrao, et al. [16] | | 0.691 | 0.633 | -0.754 | 12.28 |
| Yusuf, et al. [26] | | 0.793 | 0.662 | -0.949 | 9.75 |
| Narasimha, et al. [24] | | 0.707 | 0.555 | -0.9 | 8.01 |
| Chaudhari, et al. [18] | | 0.011 | 0.001 | -0.174 | 0.15 |
| Dutta, et al. [21] | | 0.645 | 0.568 | -0.733 | 11.26 |
| Lal, et al. [22] | | 0.402 | 0.304 | -0.531 | 7.07 |
| Makkar, et al. [15] | | 0.503 | 0.403 | -0.628 | 8.53 |
| Sawadkar, et al. [23] | | 0.594 | 0.486 | -0.727 | 9.13 |
| (REM) pooled LR | | 0.619 | 0.556 | -0.69 | - |
| Note: Heterogeneity chi-squared=39.27 (d.f=11) p=0.000; Inconsistency (I-square)=72.0 %; Estimate of between-study variance (Tau-squared)=0.0226 No. studies=12; Filter off add 1/2 to all cells of the studies with zero. | ||||
Table 5: Summary negative likelihood ratio (Random effects model) (HSS>5).
| Summary of the outcomes of the overall meta-analysis | Overall | |
|---|---|---|
| Heterogeneity | Combined Results | |
| Sensitivity | p-value <0.001 I2=90.5% | 0.41 (0.38–0.44) |
| Specificity | p-value=0.1446 I2=30.8% | 0.99 (0.98–1.00) |
| DOR | p-value=0.1539 I2=29.8% | 34.55 (14.22–83.93) |
| AUC | - | 0.0336 |
| Q | - | 0.9195 |
Table 6: Summary table of the outcomes of the overall meta-analysis.
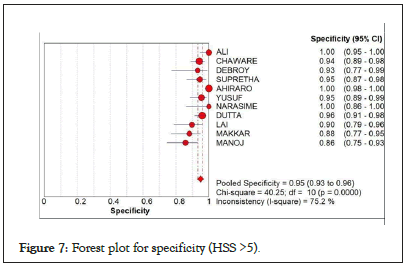
Figure 7: Forest plot for specificity (HSS >5).
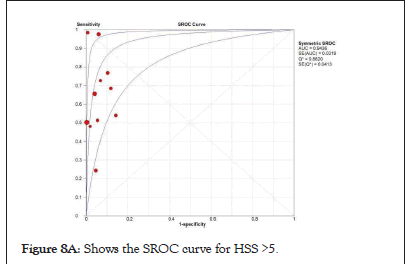
Figure 8a: Shows the SROC curve for HSS >5.
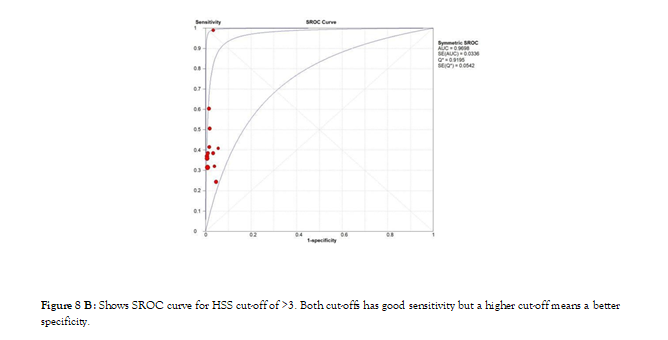
Figure 8b: Shows SROC curve for HSS cut-off of >3. Both cut-offs has good sensitivity but a higher cut-off means a better specificity.
This meta-analysis yielded 12 eligible studies for review. It provides information on the usefulness of HSS as a screening tool to diagnose neonatal sepsis after investigating a total of 1481 participants across the 12 studies with 27% (402) having a positive blood culture result. Accepting the Hematologic Scoring System (HSS) score of 3 and above as cut-off for initiation of treatment, it showed a pooled diagnostic odds ratio of 34.55 (95% CI: 14.22-83.93) backing the use of HSS for screening or as a diagnostic procedure. Furthermore, on a separate analyses, the pooled sensitivity is at 0.41 (0.38-0.44) at 95% CI and a specificity of 0.99 (0.98-1.00) at 95% CI. This further supports its ability to screen cases of neonatal sepsis and to tell if the disease is unlikely to be present. However, the studies included when pooled revealed statistically high heterogeneity. Not to get confused with the result, the study also looked into the statistical significance for when the HSS score is 5 and above, a score which Rodwell, et al. [13] identified where sepsis is highly likely. The result showed e slight decrease in sensitivity but an improved specificity.
Only 2 of the 12 included studies provided information on the prenatal and presentation of the participants upon admission. In the study of Dutta, et al. [21] numerically significant are the findings of respiratory symptoms and tachypnea as clinical manifestation of sepsis. Chaware, et al. [19] also presented feeding intolerance and vomiting as clinical presentation of sepsis. Dutta, et al. [21] also noted that premature labor and premature rupture of membrane as top two maternal factors surrounding the cases of neonatal sepsis. Other studies did not present their findings hence cannot statistically treat.
Looking at the positive and negative likelihood ratios, the study provides that having a score of 3 and above indeed increased the likelihood that a patient could have sepsis and a score lower than that is 60% likely to be negative for neonatal sepsis.
In the study of Ahirrao, et al. [16] score of >3 detected 202 out of 238 cases (84.9%) of culture positive proven sepsis and probable sepsis. Rodwell, et al. [13] Makkar, et al. [15] found 93.7%, of cases with microbiological and clinical evidence of sepsis. 36 out of 58 neonates had clinical evidence of sepsis though they had low HSS score. Thus high HSS score was more reliable predictor of sepsis than a low HSS score suggesting absence of disease. The higher the score, the greater was the certainty of sepsis being present.
The HSS should improve the efficiency of the CBC as a screening test for sepsis and permits an objective assessment of hematological changes. Analysed the hematological profiles in the light of the HSS and found that I:M ratio was the most reliable indicator in identifying infants with sepsis.
Neonatal sepsis remains to be a leading cause of morbidity and mortality especially in the middle and low-income countries to which our country belongs. And although blood culture remains to be the definitive diagnostic procedure of choice, its availability, the actual procedure, requirements and turnaround time limits its efficiency especially in low-resourced areas to even the city centers. Clinicians are usually left with their history and physical findings to rely on and a screening test that is the Complete Blood Count (CBC) that is almost negligible unless very telling as a source of basis for treatment or non-treatment. Either of the two decisions are not without its consequences as over-treatment may cause undue exposure to strong chemical agents causing death in microfloras or lead to multi-drug resistant microorganisms, or death of the child at the other end of the spectrum.
This systematic review and meta-analysis analyzed fair to good quality evidences that studied the accuracy of CBC improved with the use HSS as a screening tool for sepsis revealed that the Hematologic Scoring System (HSS) is an accurate and a reliable screening tool for the diagnosis of Neonatal Sepsis. It is a quick and economical screening tool that is objective and thus increases the weight of a regular CBC.
Mostly, the source of studies that were used in this meta-analysis usually lacks specific information on the background of the neonates; prenatal and postnatal, the time when the extraction was done and other issues surrounding a sick neonates. A more rigorous and meticulous study design such as a randomized controlled trials made in our setting are needed to further determine its accuracy in diagnosing neonatal sepsis.
The investigators encourage laboratories, when requested for possible neonatal sepsis cases, would be able to adapt this scoring system as part of their routine complete blood count result.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref]
[Crossref] [Google scholar] [PubMed]
Citation: Grandeza III NF, Resurreccion HJL (2023) Hematologic Scoring System (HSS) As a Tool for Early Diagnosis of Neonatal Sepsis: A Systematic Review and Meta-Analysis. J Hematol Thrombo Dis. 11:549.
Received: 31-May-2023, Manuscript No. JHTD-23-24628; Editor assigned: 02-Jun-2023, Pre QC No. JHTD-23-24628 (PQ); Reviewed: 16-Jun-2023, QC No. JHTD-23-24628; Revised: 23-Jun-2023, Manuscript No. JHTD-23-24628 (R); Published: 30-Jun-2023 , DOI: 10.35248/2329-8790.23.11.549
Copyright: © 2023 Grandeza III NF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.