Journal of Glycomics & Lipidomics
Open Access
ISSN: 2153-0637
ISSN: 2153-0637
Research Article - (2012) Volume 0, Issue 0
Sugar chains envelop the vast majority of the cell surface and thus have been considered to play pivotal roles in cell-to-cell and cell-to-extracellular matrix interactions. Recent studies have shown that expression of N-acetylglucosaminyltransferase (GnT) V is induced in hepatomas, and high-branched N-linked sugar chains play important roles in carcinogenesis and metastasis. However, the precise structural changes have not yet been studied in detail. Here, we compared sugar chains expressed in normal mouse liver, regenerating liver, three mouse hepatoma cell lines and Hepa 1-6-related tumors obtained by inoculation of Hepa 1-6 cells into liver or subcutaneous tissue of BALB/c nu/nu mice. The products of GnT V were found to be present in similar proportions in the mouse liver and in hepatomas, while there was a big difference in the amount of GnT IV products in these organs. Normal mouse liver and regenerating mouse liver contained small amounts of sugar chains produced by the action of GnT IV, while such chains were abundant in hepatoma cell lines and in Hepa 1-6-related tumors. Analysis of N-linked sugar chains in human livers and in hepatocellular carcinoma (HCC) revealed that both GnT IV and GnT V products are present in human liver, but their total content did not change during malignant transformation. Thus there was no increase in GnT V N-glycan products in hepatoma tissues in the mouse model or in humans.
<Keywords: Hepatocellular carcinoma; Regenerating liver; N-glycan; GnT V; GnT IV
N-linked sugar chains of glycoproteins play pivotal roles in cellto- cell and cell-to-extracellular matrix interactions. Recently N-linked sugar chains have received significant attention from a clinical perspective. Human malignant tumors including esophageal carcinoma [1], human breast cancer [2-4], colon cancer [3,5], and malignant cells isolated from patients with Sezarry syndrome [6] have been shown to express increased levels of N-glycans having ß1, 6-branching. However, these results were obtained through lectin histochemical analysis (mostly using leukoagglutinin lectin) and therefore the precise structure of these glycans has not been determined. The ß1, 6-branched structure is synthesized by ß1, 6-N-acetylglucosaminyltransferase V (GnT V) (Figure 1). Many studies have shown an increase in GnT V activity in cancer. In particular, in breast cancer cells a positive correlation was observed between GnT V activity and tumor size [2]. In human colon cancers, it was reported that the expression of GnT V correlated with a poor prognosis and distant metastasis [7]. Thus, GnT V is considered a key enzyme in cancer progression and metastasis, and its expression level as well as the levels of its reaction products may be a prognostic marker for patients.
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers [8,9]. Alpha-fetoprotein (AFP) is a glycoprotein that has an N-linked sugar chain structure, and it was reported that the relative amount of the Lens Culinaris Agglutinin (LCA)-reactive species of AFP is significantly higher in HCC than in non-neoplastic liver disease [10,11]. Furthermore, gene expression and enzyme activity of some glycosyltransferases, including GnT V, are elevated nearly 10- fold in HCC tissue [12,13]. These studies indicate that alterations in N-linked sugar chain expression should be drastic in HCC. However, little is known regarding the precise structure of N-linked sugar chains expressed in HCC cells or tissues.
We previously developed a sensitive method to systematically analyze N-linked sugar chains present in whole tissues or cells [14-16]. In the present study, we analyzed the structure of N-linked sugar chains expressed in Hepa 1-6 liver tumors and compared this to their structure in normal mouse liver. Significant differences were observed in the sugar chain structure expressed in these two types of tissue. Next, we tried to identify the structure specific to malignant transformation. Cell growth-related alterations were ruled out by analyzing the N-linked sugar chains expressed in the regenerating liver. N-linked sugar chains with higher branching (tri-antennary or tetra-antennary structure) catalyzed by GnT V (ß1, 6-branching) were expressed abundantly in normal liver, while a tri-antennary structure catalyzed by GnT IV (ß1, 4-branching) was poorly expressed. In murine hepatoma cells or tissues the amount of GnT IV products increased. In contrast, human liver contained a significant amount of GnT IV N-glycan products. In human hepatomas our results contrasted with previous reports that showed a correlation between the extent of malignancy and GnT V expression levels, and the GnT V level did not change during malignant transformation.
Materials and chemicals
Anhydrous hydrazine was purchased from Tokyo Chemical Industry (Tokyo, Japan), 2-aminopyridine was from KANTO CHEMICAL (Tokyo, Japan), and dimethylamino-borane was from Wako (Osaka, Japan). GlycoTAG was from Takara Bio (Otsu, Japan). Graphite carbon columns were purchased from GL Science (GLPak carbograph, Tokyo, Japan). Cellulose cartridge columns were from Takara Bio and GL Science. Microgranular cellulose for packed cellulose columns was from Sigma (St. Louis, MO). Neuraminidase derived from Arthrobacter ureafaciens was purchased from Nacalai Tesque (Kyoto, Japan). Pyridylaminated (PA)-sugar chains used as a standard were purchased from Takara Bio and Seikagaku Corporation (Tokyo, Japan) or kindly provided by Dr. Sumihiro Hase (Osaka University, Japan) and by Dr. Atsushi Nishikawa (Tokyo University of Agriculture, Japan).
Animals: Nine-week-old female ICR mice and BALB/c nu/nu mice were purchased from Japan SLC (Hamamatsu, Japan). All experiments were carried out with permission of the institutional Animal Research Committee of the National Institute for Physiological Sciences.
Cell lines and cell culture: The murine HCC cell line, Hepa 1-6 and the human HCC cell line, HepG2 were purchased from RIKEN CELL BANK (Tsukuba, Japan). The murine HCC cell line BNL1ME A.7R.1 was purchased from the American Type Culture Collection (Rockville, MD, USA). All cells were propagated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS (fetal bovine serum) in a humidified atmosphere of 5% CO2 at 37°C.
Specimen: The HCC tissues were obtained from patients who had agreed to participate in this study, and who received a hepatectomy in the Kagawa University Hospital. Resected HCC tissues and surrounding normal liver tissues were analyzed after formalin fixation.
Purification and pyridylamination of sugar chains: N-glycan purification and pyridylamination were performed as described previously [14-18]. Briefly, nine-week-old mice were sacrificed and their whole livers were quickly removed and washed with ice-cold PBS. Tissues were homogenized in a nine-fold volume of acetone using a polytron homogenizer. After acetone precipitation, samples were lyophilized before use. A lyophilized sample (2 mg) was hydrazinolyzed (100°C, 10 hr) and then N-acetylated. The reducing ends of the liberated glycans were tagged with the fluorophore 2-aminopyridine to aid detection in high-performance liquid chromatography (HPLC) analyses.
HPLC Analysis: Analyses of pyridylaminated (PA) sugar chains using HPLC were performed as described previously [14-18]. Briefly, PA-N-glycans were digested with excess units of neuraminidase at 37°C for 14 hr, followed by heating at 100°C for 5 min and filtering through a 0.20 μm spin filter (Ultrafree-MC LG, Millipore, Billerica, MA). To separate neutral from acidic N-glycans, PA-N-glycans were passed through an anion-exchange column (Mono Q 5/50 GL, GE Healthcare, Little Chalfont, UK) using HPLC or a Microgranular DE52-packed column (Whatman, GE Healthcare). Water adjusted to pH 9.0 with ammonia was used as a mobile solvent. Neutral N-glycans were collected in the non-adsorbed fraction. PA-tagged N-glycans of varying sizes were separated by HPLC using a Normal-phase (NP)- column (Shodex Asahipak NH2P-50 4E, 4.6×250 mm, Showa Denko K.K., Tokyo, Japan) at a flow rate of 0.6 ml/min at 30°C. PA-N-glycans were detected at an excitation wavelength of 310 nm and an emission wavelength of 380 nm using a fluorescence detector [19]. Each detected PA-N-glycan was further analyzed by reverse-phase (RP) HPLC. RPHPLC was performed on a Develosil C30-UG-5 column (4.6×150 mm, Nomura Chemical, Seto, Japan) at a flow rate of 0.5 ml/min at 30°C. PA-sugar chains were detected at excitation and emission wavelengths of 320 nm and 400 nm, respectively. N-glycan structures were identified by calculating the Mannose-Unit value from NP-HPLC, and the Glucose-Unit value from RP-HPLC, as described previously [17,19], or by comparison with known standards and sequential exoglycosidase digestion. Typical chromatograms from NP-HPLC and RP-HPLC are shown in Supplemental figure 1.
Data quantification and analysis of PA-sugar chains: PA-Nglycans were quantified and analyzed as described previously [14-18]. HPLC chromatogram data were analyzed with Unipoint software (Gilson Inc., Middleton, WI, USA), LC station software (Shimadzu) and Empower2 software (Waters, Milford, MA, USA).
Preparation of HCC cell lines and regenerating livers for sugar chain analysis: HCC cells (80 to 90% confluent) cultured in 10 cm dishes were harvested after rinsing with PBS. The HCC cells collected from 20 dishes were homogenized in acetone using a polytron homogenizer and processed as above. Three nine-week-old ICR mice were subjected to a two-thirds partial hepatectomy, and three nine-week-old ICR mice were subjected to a sham operation (only peritoneotomy). Regenerating livers and sham-operated livers were obtained 48 hr after the operation and the structures of N-linked sugar chains were analyzed as above.
Hepa 1-6 cell inoculation: In subcutaneous tumors, Hepa 1-6 cells were suspended in DMEM at a concentration of 1×107 cells/1ml, and 100 μl of this suspension was injected subcutaneously into the flank regions of three 9-week-old BALB/c nu/nu mice. The mice were sacrificed when the diameters of their subcutaneous tumors reached 2 cm, at which time the tumors were removed and the structures of N-linked sugar chains analyzed. To form liver tumors, 2×105 Hepa 1-6 cells were suspended in 200 μl of DMEM and injected under the capsules of spleens of three 9-week-old BALB/c nu/nu mice. After 4 weeks, metastatic liver tumors were removed and the structures of N-linked sugar chains were analyzed after eliminating the surrounding normal liver tissue. Some tumors were fixed in 4% paraformaldehyde and stained with hematoxylin and eosin for histopathological analysis.
Hematoxylin and Eosin (HE) staining of Hepa 1-6 liver tumor: For liver tumor formation, 2×105 Hepa 1-6 cells were suspended in 200 μl of DMEM and injected under the capsule of the spleens of three 9-week-old BALB/c nu/nu mice. After 4 weeks, metastatic liver tumors were removed, fixed in 4% paraformaldehyde, and stained with hematoxylin and eosin.
Structural analysis of major N-linked sugar chains expressed in ICR mouse liver
We first determined the structures of the major N-linked sugar chains expressed in ICR mouse liver. Acetone precipitated tissues were hydrazinolyzed, pyridylaminated and subjected to twodimensional HPLC. Nearly 20 RP-HPLC peaks were collected. Each peak was re-applied to NP-HPLC to measure the precise mannose unit value (MU). Two-dimensional maps were thus obtained of 16 pyridylaminated N-linked sugar chains expressed in ICR mouse liver with two indices, consisting of the MU measured by NP-HPLC and the glucose unit (GU) measured by RP-HPLC. Positions of major peaks on the two dimensional HPLC map corresponded to those of standard pyridylaminated sugar chains. Co-elution with the standard identified the structures of all major peaks in the normal liver. Major N-linked sugar chains (exceeding 1% of the total amount of N-glycans) expressed in ICR mouse liver and their amounts were determined and are shown in table 2 (abbreviations are shown in table 1). The liver-type features of N-linked sugar chains expressed in mouse were as follows: (1) Sugar chain structure expressed in normal liver consisted mainly of high mannose-type sugar chains (M5, M6, M7, M8, M9), A2G2 and A2G2F. (2) Tri-antennary chains expressed in the normal liver were mainly A’3G3, which is the structure with GlcNAc added to A2G2 via ß1, 6 linkage presumably by the action of GnT V (Supplemental figure 2).
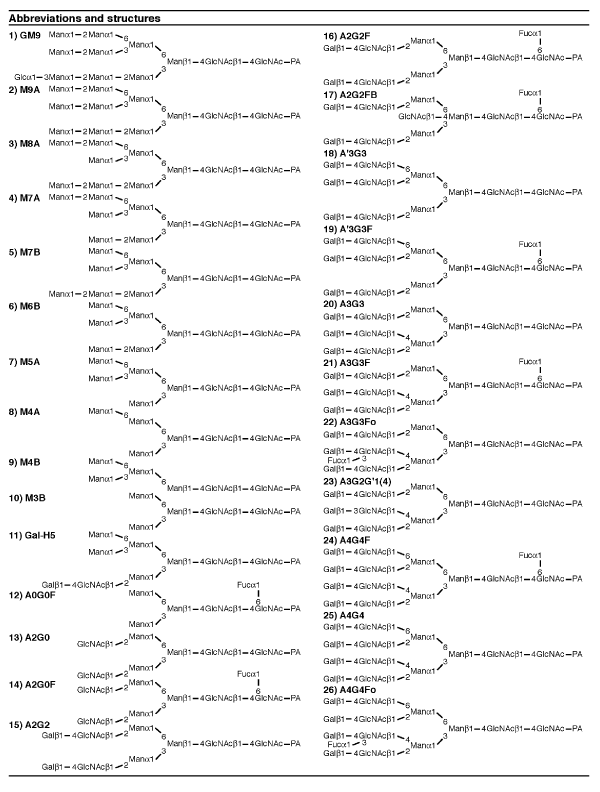 |
Table 1: Abbreviations and structures of PA-N-glycans. All structures are shown as pyridylaminated (PA-) forms. The nomenclature of the oligosaccharide structures was reported previously [16,17]. N-acetylglucosamine; GlcNAc, mannose; Man, galactose; Gal, Glucose; Glc, fucose; Fuc.
| Normal liver | Hepa 1-6 liver tumor | Hepa 1-6 subskin tumor | Hepa 1-6 | HepG2 | BNL | Sham operation | Regenerating liver | ||||||
| Structure | Mean (%) | SD | Mean (%) | SD | Mean (%) | SD | Mean (%) | Mean (%) | Mean (%) | Mean (%) | SD | Mean (%) | SD |
| High-mannose type | |||||||||||||
| GM9 | 2.3 | 0.1 | 1.1 | 0.2 | 1.3 | 0.2 | 1.1 | 2.2 | 2.1 | 1.0 | 0.4 | 1.1 | 0.0 |
| M9A | 14.0 | 0.3 | 8.9 | 0.8 | 7.6 | 0.6 | 7.8 | 8.1 | 7.7 | 14.9 | 2.3 | 15.8 | 0.7 |
| M8A | 15.9 | 0.8 | 10.4 | 0.6 | 10.7 | 0.5 | 9.7 | 18.0 | 7.4 | 16.1 | 4.0 | 14.9 | 1.1 |
| M7A | 3.3 | 0.5 | 2.5 | 0.2 | 5.5 | 1.1 | 5.4 | 3.9 | 4.6 | 3.0 | 0.3 | 2.9 | 0.2 |
| M7B | 4.0 | 0.4 | 2.6 | 0.4 | 3.4 | 0.9 | 2.7 | 4.2 | 2.0 | 3.0 | 1.3 | 2.7 | 0.2 |
| M6B | 7.4 | 0.6 | 6.4 | 0.6 | 5.5 | 0.6 | 8.0 | 5.3 | 9.6 | 8.4 | 1.4 | 8.8 | 0.4 |
| M5A | 5.7 | 0.9 | 4.9 | 0.6 | 6.0 | 0.2 | 4.1 | 7.3 | 3.9 | 6.3 | 1.7 | 7.3 | 0.8 |
| Hybrid type | |||||||||||||
| Gal-H5 | ND | - | ND | - | 3.6 | 0.5 | 5.4 | 2.9 | 1.6 | ND | - | ND | - |
| Complex type | |||||||||||||
| A2G0 | 1.3 | 0.4 | ND | - | ND | - | ND | ND | ND | 1.4 | 0.5 | 1.7 | 0.3 |
| A2G0F | 1.5 | 0.4 | ND | - | ND | - | ND | ND | ND | 1.0 | 0.7 | 1.3 | 0.2 |
| A2G2 | 13.4 | 0.2 | 24.1 | 1.8 | 8.5 | 0.5 | 7.7 | 5.2 | 6.9 | 15.2 | 1.4 | 19.0 | 1.3 |
| A2G2F | 4.3 | 1.2 | 8.9 | 0.3 | 8.2 | 0.5 | 10.1 | 7.2 | 6.4 | 4.4 | 2.0 | 4.8 | 0.5 |
| A2G2FB | ND | - | 0.6 | 0.0 | 1.9 | 0.2 | 1.1 | ND | ND | ND | - | ND | - |
| A'3G3 | 3.3 | 0.3 | 5.4 | 0.7 | 2.9 | 0.2 | ND | ND | ND | 4.1 | 1.0 | 5.4 | 0.8 |
| A3G3 | ND | - | 0.6 | 0.1 | 1.3 | 0.2 | 5.3 | 3.2 | 4.4 | ND | - | ND | - |
| A3G3F | ND | - | 0.5 | 0.1 | 1.1 | 0.3 | 1.3 | ND | ND | ND | - | ND | - |
| A3G2G'1(4) | ND | - | ND | - | ND | - | 2.5 | 1.3 | 3.3 | ND | - | ND | - |
| A4G4F | ND | - | 0.4 | 0.1 | 1.1 | 0.2 | 2.1 | 2.0 | ND | ND | - | ND | - |
| Others | |||||||||||||
| M4A | 0.9 | 0.2 | ND | - | ND | - | ND | ND | ND | 0.7 | 0.2 | 0.5 | 0.1 |
| M4B | 1.8 | 0.5 | 1.1 | 0.2 | 2.2 | 0.3 | ND | 1.9 | ND | 1.1 | 0.2 | 1.4 | 0.3 |
| M3B | 2.2 | 0.4 | 1.4 | 0.1 | 2.9 | 0.2 | 2.0 | 5.6 | 1.6 | 1.6 | 0.2 | 2.1 | 1.0 |
| A0G0F | 1.1 | 0.4 | 1.6 | 0.3 | ND | - | 2.7 | ND | ND | 1.9 | 1.4 | 0.9 | 0.2 |
| GnT V products | 3.3 | 0.3 | 5.8 | 0.8 | 4.0 | 0.4 | 2.1 | 2.0 | ND | 4.1 | 1.0 | 5.4 | 0.8 |
| GnT IV products | ND | - | 1.5 | 0.3 | 3.5 | 0.7 | 11.2 | 6.5 | 7.7 | ND | - | ND | - |
Table 2: N-glycans expressed in mouse normal livers, hepatomas, regenerating livers, and in liver metastasized or subcutaneously transplanted Hepa 1-6 cells or HCC cell lines. Amounts of major N-linked sugar chains expressed in normal or regenerating livers of three 9W mice together with those from three sham operated mice were measured. The amounts of N-linked sugar chains exceeding 1% of the total amounts did not vary significantly among the samples, and the standard deviations (SD) of each sugar chain level were extremely small. Amounts of N-linked sugar chains expressed in liver metastasized or subcutaneously transplanted Hepa 1-6 cells were analyzed, together with Hepa 1-6, BNL and HepG2 hepatoma cell lines in culture. ND, not detected.
To study variation in expression patterns among individuals, amounts of major N-linked sugar chains expressed in livers of three 9W mice were measured. The amounts of N-linked sugar chains exceeding 1% of the total amount did not vary significantly among the samples, and the standard deviation (SD) of each sugar chain level was extremely small. This indicates that the expression of N-linked sugar chains is strictly regulated in the mouse liver so that there are few differences among individuals.
Structural analysis of major N-linked sugar chains expressed in Hepa 1-6 liver tumor
We next investigated the sugar chain structural changes in Hepa 1-6 tumors metastasized in liver. We injected 2×105 Hepa 1-6 cells into spleen of BALB/c nu/nu mice to make liver tumors ranging from 2 mm to 8 mm after about 4 weeks. HE staining of a representative tumor is shown in figure 2. From the HE staining, the tumor-rich area that was dissected out seemed to consist mainly of tumor tissue with little normal liver contamination. Three tumors were obtained from the same mouse liver. Metastatic tumor formation was confirmed in the liver by histopathological analysis (data not shown). The tumors were freed of surrounding normal liver tissue and then homogenized together. Sugar chains from tumors from three mice were analyzed and the results are shown in table 2 (Hepa 1-6 liver tumor).
Figure 2: HE staining of Hepa 1-6 liver tumor. (A) For liver tumor formation, Hepa 1-6 cells were suspended in DMEM and injected under the capsule of the spleen in three 9-week-old BALB/c nu/nu mice. After 4 weeks, metastatic liver tumors were removed, fixed in 4% paraformaldehyde, and stained with HE. Scale bar, 250 μm (B) High-power field. Scale bar, 100 μm.
A number of significant changes were observed: 1) Disappearance of A2G0, A2G0F and M4A. 2) Appearance of A2G2FB, A3G3, A3G3F and A4G4F. Those that disappeared were presumably degradation products, suggesting rapid reutilization of glycoproteins in liver tumors compared with normal liver. Appearance of A3G3, A3G3F and A4G4F was interesting because all of these sugars utilize GnT IV for their biosynthesis (Supplemental figure 2).
Structural analysis of major N-linked sugar chains expressed in subcutaneous tumor and in HCC cell lines
We wanted to understand the cause of the observed changes and determine whether they were due to the nature of transformation or to the presence of extracellular cues. Hepa 1-6 cells (1×106) were injected subcutaneously into the flank regions of three 9-week-old BALB/c nu/ nu mice, and subcutaneous tumors were formed. Three subcutaneous tumors were homogenized and the sugar chain structures were analyzed separately. The mean relative abundance of sugar chain structures expressed in three subcutaneous tumors is shown in table 2 (Hepa 1-6 subskin tumor). All of the changes observed in the liver tumor were reproduced in the subcutaneous tumor. A characteristic feature was expression of Gal-H5, a hybrid type sugar chain.
The amounts of N-glycans expressed in Hepa 1-6 and other hepatoma cell lines, BNL and HepG2, in culture were analyzed. A2G0, A2G0F and M4A were not detected in these cells, while A2G2FB, A3G3, A3G3F and A4G4F newly appeared (Table 2). In all the cell lines the total amount of sugar chains that require the action of GnT IV for their biosynthesis (such as A3G3 and A3G2G’1(4)) was high, reproducing the findings from the previous experiments (Supplemental figure 2).
Structural analysis of major N-linked sugar chains expressed in regenerating liver
The difference in sugar chain expression patterns observed above may be related to malignant transformation. However, it is also possible that these changes merely reflected changes in the cell proliferation rate (normal liver cells rarely divide, while tumor cells divide rapidly). To check this possibility, we next analyzed the structure of major N-linked sugar chains expressed in the regenerating liver, which is proliferating rapidly, and also in sham-operated mouse liver. The results are shown in table 2 and in supplemental figure 2 (“Sham operation” and “Regenerating liver”). Sugar chains expressed in the regenerating liver and sham operated liver were similar to those in the normal liver, except for the increase in the amount of A’3G3 in the regenerating liver, which may reflect an increase in the GnTV m-RNA level during liver regeneration in rat [20]. This suggested that alterations of sugar chain expression patterns in HCC from the normal liver are only weakly related to their different proliferation rates.
Structural analysis of major N-linked sugar chains expressed in human liver
Non-pathological regions of eight tumor-bearing patient livers dissected during the operation were analyzed. The overall expression pattern of N-linked sugar chains was very similar to that of mouse liver. The high mannose type sugar chains A2G2 and A2G2F were abundant (data not shown). However, analysis of tri- and tetra-antennary sugar chains revealed that human livers contain more GnT IV products (such as A3G2G’1, A3G3F and A3G3Fo) than GnT V products (such as A’3G3 and A’3G3F) (Table 3), which was totally different from the sugar chain content in the mouse liver (Table 2). Among the eight samples the amount of tri- and tetra-antennary sugar chains was very similar (Table 3). These liver regions did not contain tumor tissue, and their morphology appeared to be normal. We presume that the sugar chain contents in these samples are close to those of normal liver.
| Structure | Control 1 | Control 2 | Control 3 | Control 4 | Control 5 | Control 6 | Control 7 | Control 8 | Hepatoma 1 | Hepatoma 2 | Hepatoma 3 | Hepatoma 4 |
| A4G4 | 0.42 | 0.18 | 0.41 | 0.57 | 0.55 | 0.49 | 0.50 | 0.41 | 0.41 | 1.13 | 0.64 | 0.13 |
| A4G4F | 0.23 | 0.35 | 0.33 | 0.39 | 0.42 | 0.26 | 0.27 | 0.21 | 0.54 | 0.10 | 0.17 | 0.28 |
| A4G4Fo | 0.19 | 0.10 | 0.14 | 0.11 | 0.15 | 0.10 | 0.40 | 0.10 | 0.08 | 0.10 | 0.02 | 0.28 |
| A3G3 | 0.06 | 0.04 | 0.05 | 0.01 | 0.02 | 0.08 | 0.04 | 0.07 | 0.03 | 0.07 | 0.07 | 0.02 |
| A3G2G'1(4) | 1.40 | 1.18 | 1.70 | 2.21 | 1.57 | 1.67 | 0.97 | 1.24 | 0.38 | 1.18 | 3.15 | 0.80 |
| A3G3F | 0.88 | 0.73 | 0.77 | 0.38 | 0.56 | 0.62 | 0.47 | 0.39 | 1.00 | 0.19 | 0.29 | 0.73 |
| A3G3Fo | 0.92 | 1.04 | 0.87 | 0.61 | 0.54 | 0.60 | 0.63 | 0.74 | 0.87 | 0.17 | 0.33 | 0.49 |
| A'3G3 | 0.34 | 0.17 | 0.38 | 0.48 | 0.16 | 0.27 | 0.43 | 0.33 | 0.33 | 0.64 | 0.19 | 0.39 |
| A'3G3F | 0.55 | 0.53 | 0.51 | 0.32 | 0.32 | 0.38 | 0.27 | 0.45 | 0.31 | 0.12 | 0.23 | 0.76 |
| GnT V products | 1.72 | 1.33 | 0.89 | 1.88 | 1.61 | 1.50 | 1.88 | 1.44 | 1.69 | 2.10 | 1.26 | 1.84 |
| GnT IV products | 4.10 | 3.63 | 4.27 | 4.29 | 3.83 | 3.82 | 3.28 | 3.17 | 2.42 | 2.94 | 4.68 | 2.72 |
Table 3: Amount of triantennary and tetraantennary N-glycans expressed in human hepatocellular carcinomas and non-pathological regions of human liver. N-glycan expressed in non-pathological regions of eight tumor-bearing patient livers dissected during the operation (Control) and four dissected hepatocellular carcinomas were analyzed. The table shows the amounts of triantennary and four antennary N-glycans expressed in these tissues in terms of the percentages of the total amount of N-glycans.
Analysis of Tri- and Tetra-antennary sugar chains in human hepatoma tissues
The amount of tri- and tetra-antennary sugar chains expressed in hepatoma tissues (4 primary hepatocarcinoma tissues (Hepatoma 1 to 4)) was analyzed next. The amount of GnT IV or GnT V N-glycan products in hepatoma was not altered significantly from that in nonpathological regions of the liver (Table 3, supplemental figure 3).
Expression of N-linked sugar chains has been considered to undergo dramatic changes during malignant transformation (see Introduction). However, previous studies have not detected structural changes in N-linked sugar chain expression patterns in tissues, but rather focused on sugar chain alterations on a particular protein. Such changes may not reflect the overall change in N-glycan expression patterns caused by changes in glycosyl transferase gene expression. In many studies N-glycan expression in several cancer tissues has been shown to be increased using lectin staining [1-5], in which any information regarding structural alterations was limited. In the present study, N-linked sugar chains were released from tissues and the N-linked sugar chain expression pattern was analyzed systematically. Levels of N-linked sugar chains produced by the action of GnT IV, but not V, were found to be increased in mouse hepatoma. The GnT V products did not increase in human primary hepatocarcinoma tissues compared to non-pathological regions. Discrepancy in the level of GnT V products detected by the two methods (lectin staining and chemical analysis) could be that the lectin used (L-PHA in most cases) can react with sugar chains other than GnT V products. It is also possible that some of the GnT V products are lost during our purification procedure although we have so far never encountered such N-glycans.
Involvement of GnT IV and GnT V during hepatic tumor formation
GnT IV and V catalyze the substitution of the trimannosyl core with a ß1,4- or ß1,6-linked N-acetylglucosamine residue, respectively, and are key enzymes in the processing of multiantennary N-linked sugar chains during the synthesis of glycoproteins. Recent experiments have revealed the mechanisms by which oncogenesis cause changes in GnT V activity. GnT V transcription is stimulated by several oncogenes including src, her-2/neu, H-ras, and v-sis [21-23] and this overexpression is regulated through the ras-raf-ets pathways. Stimulation of GnT V activity has also been observed via the protein kinase C and phosphatidylinositol 3-kinase-protein kinase B signaling pathways [21-25]. These studies demonstrated that up-regulation of the GnT V gene is closely related to tumorigenesis and may play an important role in this process. The N-linked sugar chains detected in this study that are produced by the action of GnT V are A’3G3 and A4G4F, whereas A3G3, A3G3F and A3G2G’1(4) are the products of GnT IV catalysis. However, the proportion of sugar chains synthesized by GnT V was greater in the normal and regenerating mouse livers than in mouse HCC tumors or in cell lines and, moreover, they were not detected in BNL cells. On the other hand, a clear increase in the proportion of GnT IV products was observed in mouse tumors. These sugar chains were below the detection level (0.5%) in the normal or regenerating mouse liver. This result contradicts previous studies demonstrating a clear correlation between the GnT V-mRNA level and malignant transformation. Also, in humans the amount of hepatoma GnT V products did not increase (Table 3, supplemental figure 3). It has been reported that one of the GnT V gene products is secreted and induces tumor angiogenesis [26]. Increased GnT V gene expression may contribute more significantly by increasing this activity than by increasing the 1,6-branching of N-glycan structures.
The authors are grateful to Dr. Kazuhiro Tanabe for helpful discussions and Ms. Isoko Itoh and Takako Koike for their excellent technical assistance. This work was supported by Grants-in-Aid for Scientific Research of Japan.