Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
+44 1300 500008
ISSN: 2167-0412
+44 1300 500008
Research Article - (2023)Volume 12, Issue 4
Brassicaceae, one of the prime plant family encompasses the important genus-Brassica with about 37 species, including major vegetable species cultivated globally and consumed worldwide as a part of diet either raw or processed. The cruciferous vegetables hold a central position as food crops in China, Japan, India, and Europe. In India these vegetables are extensively grown in Jammu and Kashmir which is located in the North-western region of Himalayas Vegetables like broccoli, cabbage, cauliflower, kale, knol-khol and other cruciferous vegetables included in this family are rich in nutritional, antioxidant and bioactive compounds. The intake of these vegetables has often been linked to positive health benefits owing to the rich nutritional and phytochemical profile. Glucosinolate, a plant secondary metabolite and its breakdown products, commonly found in these vegetable crops have potential health benefits. Out of the 130 glucosinolates identified so far nearly 30-40 have been found in Brassica species. Isothiocyanate, the chief breakdown product of glucosinolates has been recognised for its anti-carcinogenic potential. The glucosinolate content of cruciferous vegetables is greatly influenced by various pre-harvest factors such as stage of plant at the time of harvest, environmental conditions and agricultural practices. Once harvest, these vegetable crops are subjected to different processing conditions such as boiling, blanching, frying, fermentation etc. which affect their glucosinolate content. This review aims to give an overview of the factors influencing the glucosinolate content in genus Brassica, the nutritional and phytochemical composition with the main focus on health-promoting activities.
Glucosinolate; Anti-Cancerous; Ascorbigen; Brassica; Bioactive compounds; Vegetables
Brassicaceae is one of the largest angiosperm plant families, also termed Cruciferae, which comprises 338-360 genera and about 3709 species. The International Code of Botanical Nomenclature (ICBN) vide Art. 18.5 (Vienna Code) has approved the use of both Brassicaceae and Cruciferae as names for this family [1]. This plant family is immensely endowed with species having a wide array of economic importance owing to their nutritional, medicinal and pharmaceutical potential. The Cruciferous family has its origin in the Eocene epoch in the floristic Irano-Turanian region about 37 million years ago [2]. The vegetables of the cruciferous family have been cultivated since ancient times, however, it is difficult to trace their exact history. The Sanskrit record about the cultivation of Brassica crops in India dates back to 3000 BC. Some historical records also suggest that the cabbage cultivation dates back to 8000 years ago along the coastal line of Europe [3]. The plant species belonging to this family are cultivated worldwide except Antarctica. Brassica forms one of the most important and essential genera of the family Brassicaceae while Draba, Cardamine, Erysimum, Lepidium and Alyssum belong to the other genera of this family [4]. Many controversies are surrounding the taxonomy of the Brassicaceae family and reliable scientific information related to this family is difficult to find and often questionable. Kiefer. et al. have recently come up with a database named as BrassiBase which has a comprehensive compilation of the taxonomy and systematics of the entire cruciferous family [5]. The genus Brassica encompasses around 37 species that are extensively grown globally as vegetables, forage, oilseeds and herbs of medicinal value [6]. Brassica vegetables form an important part of the human diet and are consumed by people globally. These vegetable crops hold a prime position as food crops in China, Japan, India, and Europe [7]. Table 1 summarizes the species of genus Brassica.
| Species | Variety/ subspecies/cultivar | Common name | Edible part | Reference |
|---|---|---|---|---|
| Brassica oleracea | var. capitata F. Alba | White cabbage | Leaves | (Šamec, et al.) [122] |
| var. capitata F.rubra | Purple or red cabbage | Leaves | (Volden, Borge, et al.) [123] | |
| var. italica | Broccoli | Inflorescence | (Dhiman, et al) [124] | |
| var. gemmifera | Brussels sprouts | Buds | (Dixon) [8] | |
| var. botrytis | Cauliflower | Inflorescence | (Branca) [125] | |
| var. Acephala | Kale | Leaves | (Šamec, et al) [126] | |
| var. viridis | Collard greens | Leaves | (Šamec, et al & Salopek-Sondi, et al) [3] | |
| var. sabellica L. | Curly kale | Leaves | (Hahn et al.) [127] | |
| var. gongylodes | Kohlrabi, stem turnip | stem | (Kalloo) [128] | |
| Brassica rapa | ssp. rapa | Turnip | Root | (Nawaz et al.) [15] |
| ssp. pekinensis | Chinese cabbage | Leaves | (Dixon) [8] | |
| ssp. narinosa (or rosularis) | Asian greens, tai cai | Leaves | (Sikorska-Zimny & Beneduce) [32] | |
| ssp. chinensis | Bok choy, pak choy | Leaves | (Raza et al.) [6] | |
| ssp. nipposinica | Mizuna | Leaves | (Šamec, et al & Salopek-Sondi, et al) [3] | |
| ssp. parachinensis | Rapini, broccoli rabe, fake Chinese cabbage | Leaves, stem, flower, buds | (Sikorska-Zimny, et al & Beneduce, et al) [32] | |
| Brassica juncea | czern | Brown mustard, Indian mustard | Leaves, seeds | (Mohan Yadav et al.) [129] |
| Brassica nigra | Black mustard | Seeds | (Rahman et al.) [130] | |
| Brassica carinata | Ethiopian mustard | Leaves, seeds | (Rahman et al.) [130] | |
| Brassica napus | var. napobrassica | Rutabaga (swede) | Root | (Raza et al.) [6] |
| var. pabularia | Siberian kale | Leaves | (Raza et al.) [6] |
Table 1: Economically important species of genus Brassica
The two species of the Brassicaceae family namely Brassica oleracea and Brassica rapa are extensively grown and consumed throughout the globe as important vegetables while the seeds of Brassica nigra, Brassica carinata, and Brassica juncea are used as a condiment [3]. The statistical data of FAO indicates that oilseeds obtained from Brassica plants such as B. napus, B. rapa, B. juncea and B. carinata form 12% of the global edible vegetable oil supply. B. oleracea which includes headed and non-headed cabbages, kale, broccoli, Brussels sprouts, cauliflower and collard greens, are the major vegetables of Brassica group. Various research investigations suggest that the headed cabbages have evolved from the wild non- headed cabbage varieties having their origins in the Eastern Mediterranean and the Baltic Sea coast. There exists a vast genetic diversity within the Cruciferae family which led to the cultivation and propagation of morphologically unique crops with desirable characteristics from wild counterparts [8]. The B. oleracea cultivars are categorized into different groups based on morphology and developmental differences. The seven cultivars of B. oleracea include “capitata group”, “acephala group”, “alboglabra group”, “botrytis group”, “Italica group”, “gemmifera group” and “gongylodes group” [3]. The capitata group encompasses different headed varieties of cabbage, acephala group includes leafy green vegetables namely kale and collard greens, alboglabra group has Chinese broccoli in it and botrytis group has cauliflower, Romanesco broccoli, and broccoflower as its representatives. Broccoli is the only member of Italica group whereas Brussels sprouts and knol khol represent gemmifera and gongylodes group respectively [9]. The cruciferous vegetables are known as cool-season crops as they are capable to withstand light to moderate freezes and sometimes even brief heavy freezes as well [10]. The cruciferous vegetables also referred to as ‘cole crops’ are extensively grown in the Jammu and Kashmir, which is a cold region located in the north-western region of Himalayas [11]. The commonly cultivated varieties of Brassica vegetables in Jammu and Kashmir are presented in Table 2. Kale (Brassica oleracea var. acephala) has not been extensively used as a vegetable crop on a commercial scale in India. However, it is grown for commercial use in Kashmir and to a limited extent in the regions including Jammu, Assam and Himachal Pradesh. Kale forms an intrinsic part of the diet of people of Jammu and Kashmir and is regionally known as ‘Haak’, which seems to have evolved from a Sanskrit term ‘Shak’. Various, traditional genotypes of kale cultivated in Kashmir have been reported in the literature. These include Khanyari, Kawdari, GM Dari Haenz Haak, Wantipori Haak, Jumadari Haak, Anchari Haak and Koker Haak. Apart from the mentioned varieties, two wild genotypes namely Wappal and Pumb Haak have also been reported in the literature [12-14].Central Institute of Temperate Horticulture (CITH) of ICAR located in Rangreth, Budgam region of Kashmir has maintained germplasm for evaluation, characterization and future use. The institute has identified 10 elite varieties of Kale suitable for commercial production namely CITH- KC-07, CITH-KC-08, HW-5, CITH-KC-05, NW-SAG-36, CITH-KC-11, CITH-KC-SEL-1, CITH-KC-16, CITH-KC-26, CITH-KC-28. The institute is actively engaged in the breeding of cole crops especially cabbage to enhance its nutritional and nutraceutical potential.
| Vegetable | Variety | Characteristic features | Parts consumed | Picture |
|---|---|---|---|---|
| Cabbage (Brassica oleracea var. capitata) | Whole head cooked as vegetable, rarely fermented |  |
||
| Golden acre | Compact with few Cup shaped, small leaves, uniform heads with solid round interior |  |
||
| Pusa drum head | Wide frame, light green leaves with prominent venation. Uniform light green flat heads | |||
| Cauliflower (Brassica oleracea var. botrytis) | Curds cooked as vegetables, occasionally fermented | |||
| Snow ball | Compact, snow white medium sized curds |  |
||
| Snow ball-16 | Compact, snow white curds with raised center |  |
||
| Pusa katki | Medium sized, compact, white curds, bluish green leaves |  |
||
| Knol Khol (Brassica oleracea var. gongylodes) | Leaves, petiole and knob cooked as vegetable. Also fermented as pickle | |||
| Early white Vienna | Medium green foliage, globular, round knob |  |
||
| Purple Vienna | Purplish leaves, large globular, round knob |  |
||
| Kale(Brassica oleracea var. acephala) | Leaves cooked as vegetables | |||
| Leaves and petiole also used for fermentation | ||||
| G. M. Dari | 30-35 cm height, Condensed leaves, frost tolerant | |||
| Khanyari | 50-65 cm height, slightly puckered leave, frost intolerant |  |
||
| Kawdari | 61 cm height, highly puckered leaves, frost intolerant | |||
| Kashmiri Haak (Haenz Haak) | 45-50 cm height, narrow leaves, hardy and frost resistant |  |
||
| Anchar Haak | 48-55 cm height, purplish green or green leaves, susceptible to frost |  |
Table 2: Commonly grown cruciferous vegetables in Kashmir Himalayas
Nutritional and phytochemical composition
The vegetables belonging to the Brassicaceae family are rich in nutritional (proteins, carbohydrates and vitamins) and bioactive components [15]. Fructose, glucose and sucrose are the major soluble sugars found in Brassica. These vegetables are also noteworthy for their extremely low (approximately less than 1%) fat content and good mineral profile. Phosphorus, sodium, potassium iron, copper, selenium, calcium and zinc are among the prominent elements found in these crops. The nutritional composition of common cruciferous vegetables is depicted in Table 3. Antioxidants, the compounds associated with the prevention of oxidative stress and reduction of oxidative damage to foods and living organisms are widely found in Brassica species. The cruciferous vegetables are known to possess antioxidant potential owing to the fair amount of antioxidant compounds particularly Vitamin C, tocopherol and carotenoids along with various antioxidant enzymes such as catalase, Superoxide Dismutase (SOD) and peroxidase [16]. The phytochemicals found in plants are present in fairly good quantities in Brassica vegetables. Various researchers have reported the presence of several different classes of phytochemicals in Brassica including phenolic acids, anthocyanins, flavonols, flavones and sulfurous compounds-glucosinolates. The predominant polyphenol classes found in Brassica species include hydroxycinnamic acids, quercetin, kaempferol and isorhamnetin derivatives. The anthocyanin content has been found to vary significantly among cultivars, species and even plants of the same species, with cyanidin 3-O(sinapoyl)(feruloyl)diglucoside-5-O-glucoside and cyanidin 3-O-(sinapoyl)(sinapoyl) diglucoside-5-Oglucoside as the major anthocyanins in these crops. The other important bioactive components present in cruciferous vegetables are phytosterol and terpenoids, known for their chemopreventive and cardioprotective potential [17,18].
| Parameters | Mustard greens | Turnip | Kale | Cauliflower | Cabbage | Brussels sprouts | Broccoli |
|---|---|---|---|---|---|---|---|
| Water (g/100g) | 90.7 | 91.87 | 84.04 | 92.07 | 92.18 | 86 | 89.3 |
| Protein(g/100g) | 2.86 | 1.17 | 3.28 | 1.92 | 1.53 | 3.38 | 2.82 |
| Fat (g/100g) | 0.42 | 0.13 | 0.93 | 0.28 | 0.1 | 0.3 | 0.37 |
| Carbohydrate(g/100g) | 4.67 | 6.43 | 8.75 | 4.97 | 5.8 | 8.95 | 6.64 |
| Fiber (g/100g) | 3.2 | 1.8 | 3.6 | 2 | 2.5 | 3.8 | 2.6 |
| Sugar (g/100g) | 1.32 | 3.8 | 2.26 | 1.91 | 3.2 | 2.2 | 1.7 |
| Minerals | |||||||
| Calcium (mg) | 115 | 30 | 150 | 22 | 40 | 42 | 47 |
| Iron (mg) | 1.64 | 0.3 | 1.47 | 0.42 | 0.47 | 1.4 | 0.73 |
| Magnesium (mg) | 32 | 11 | 47 | 15 | 12 | 23 | 21 |
| Phosphorus (mg) | 58 | 27 | 92 | 44 | 26 | 69 | 66 |
| Potassium(mg) | 384 | 191 | 491 | 299 | 170 | 389 | 316 |
| Sodium(mg) | 20 | 67 | 38 | 30 | 18 | 25 | 33 |
| Zinc(mg) | 0.25 | 0.27 | 0.56 | 0.27 | 0.18 | 0.42 | 0.41 |
| Selenium (μg) | 0.9 | 0.7 | 0.9 | 0.6 | 0.3 | 1.6 | 2.5 |
| Vitamins | |||||||
| Vitamin C (mg) | 70 | 21 | 120 | 48.2 | 36.6 | 85 | 89.2 |
| Thiamine(mg) | 0.08 | 0.04 | 0.11 | 0.05 | 0.061 | 0.139 | 0.071 |
| Riboflavin (mg) | 0.11 | 0.03 | 0.13 | 0.06 | 0.04 | 0.09 | 0.117 |
| Niacin(mg) | 0.8 | 0.4 | 1 | 0.507 | 0.234 | 0.745 | 0.639 |
| Vitamin A(IU) | 3024 | 0 | 9990 | 0 | 98 | 754 | 623 |
| Vitamin E (mg) | 2.01 | 0.03 | 1.54 | 0.08 | 0.15 | 0.88 | 0.78 |
Table 3: Nutritional composition of common cruciferous vegetables as per USDA
Glucosinolates
Glucosinolates, sulfur-containing precursors of isothiocyanate, form a large group of secondary metabolites identified in some plant species including the family Brassicaceae [19]. Nearly 130 glucosinolates have been identified so far out of which 30- 40 are mostly found in Brassica species [20]. The structure of glucosinolates consists of a β-d-glucopyranose unit joined to a hydroximinosulfate ester by a sulfur bridge and a side chain. Figure 1 represents the general structure of glucosinolates. The side chain is variable and derived from eight amino acids namely alanine, valine, leucine, isoleucine, methionine, tyrosine, phenylalanine, and tryptophan. Depending on the side chain, glucosinolates are mainly of the following types:
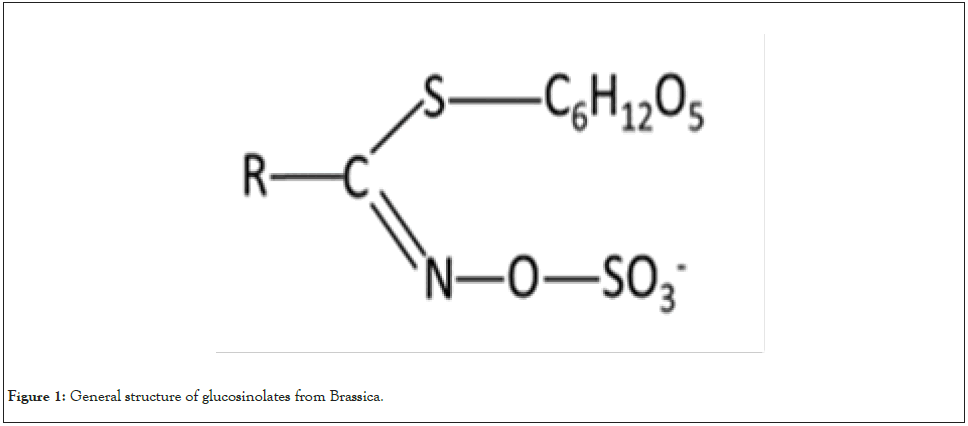
Figure 1: General structure of glucosinolates from Brassica.
Aliphatic Glucosinolates, wherein the side chain is derived from methionine, isoleucine, leucine, or valine
Aromatic Glucosinolates, wherein the side chain is derived from phenylalanine or tyrosine
Indole Glucosinolates, wherein the side chain is derived from tryptophan [21]
Although the categorization of glucosinolates as aliphatic, aromatic, and indole glucosinolates is common, it appears to be of slight biological and chemical importance and contradicts the chemical definition of the term aromatic because the indole ring system is aromatic. Blažević, et al. reviewed certain criteria for classification of glucosinolate [22]. A classification based on the precursor amino acid appears to be a more meaningful one, for example glucosinolates derived from tryptophan, glucosinolates derived from methionine, and so on. Furthermore, when assessing and discussing the physiological and ecological effects of glucosinolates, a classification based on the kind of breakdown product produced seems to be relevant.
Biosynthesis
The core biosynthesis process is common to all glucosinolates and is broadly divided into three steps as presented in Figure 2. The first step involves the chain elongation of precursor amino acid molecules and the addition of methylene groups. This step is however not seen in the case of biosynthesis of indole glucosinolates. This is followed by the formation of a core structure. The final step in the process is the modification of side chains. The side chain modification entails various chemical reactions such as oxidation, esterification, methylation, and hydroxylation [23]. During the chain elongation which consists of five enzymatic steps, the amino acids (methionine, glutamate, leucine, isoleucine and phenylalanine) undergo deamination under the action of Branched-Chain Aminotransferase (BCATs) to form the analogous 2-oxo acid which further undergoes three successive reactions in which the 2 oxo acid is first condensed with acetyl-CoA by the action of Methylthioalkylmalate synthase (MAMs) followed by an isomerization reaction with Isopropylmalate Isomerase (IPMI) as the main enzyme in action. Finally, an Isopropylmalate Dehydrogenase (IPMDHs) catalyzed oxidative decarboxylation takes place. The 2-oxo acid is elongated by a single methylene group after undergoing the above-mentioned three successive transformation reactions. The elongated molecule either enters the core structure formation pathway or goes through another chain elongation process [24,25]. The chain elongation step is followed by a core glucosinolate structure formation process consisting of seven enzymatic steps. The elongated amino acids are converted to aldoximes under the action of cytochrome P450 monooxygenases (CYP79) which are further oxidized by cytochrome P450 monooxygenases (CYP83) to form aci- nitro compounds. These aci-nitro compounds are converted to thiohydroximate and S-alkyl-thiohydroximate by enzymes namely phi and tau Glutathione S-transferases (GSTF and GSTU) and carbon-sulfur lyase (SUR1). The thiohydroximates are acted upon by uridine diphosphate glycosyltransferase 74 (UGT74) and Sulfotransferases (SOT) leading to glycosylation and sulfation to form the core structure of glucosinolate. The last step of the biosynthesis process involves the modification of side chains via various processes such as oxidation, hydroxylation, methoxylation, alkenylation, and benzoylation. For instance, the side chain modification of aliphatic glucosinolates takes place by S-oxygenation carried out by flavinmonooxygenase to form methylsulfinylalkyl glucosinolate [26,27].
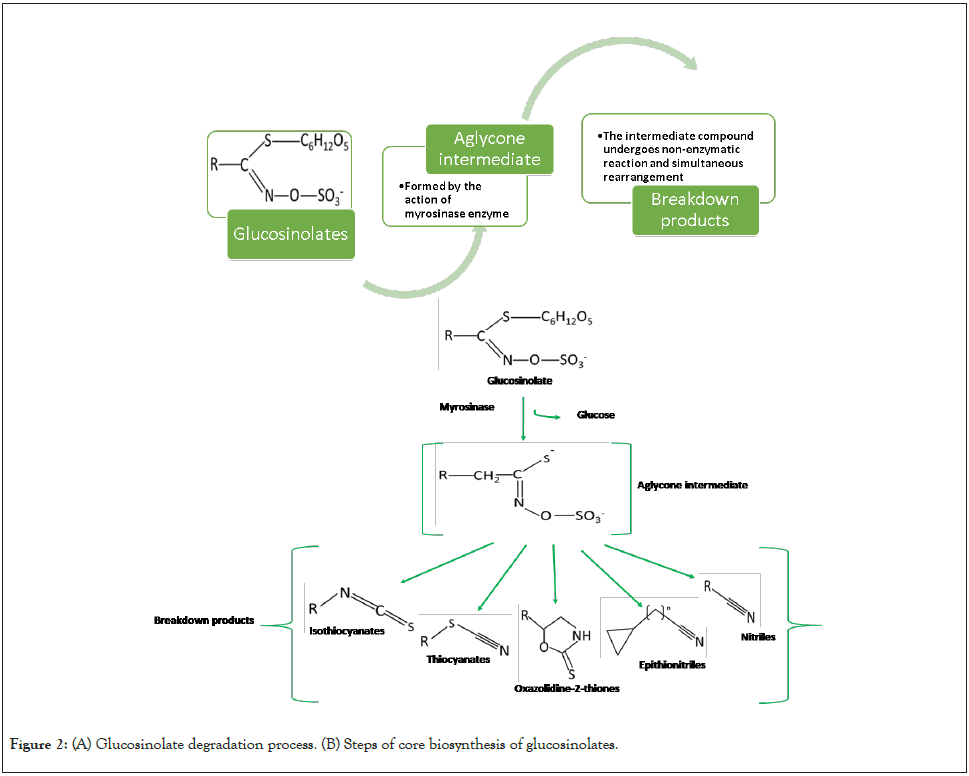
Figure 2: (A) Glucosinolate degradation process. (B) Steps of core biosynthesis of glucosinolates.
The biosynthesis of indole glucosinolates is similar to aliphatic ones though the chain elongation step is omitted. The biosynthesis pathway starts with the conversion of the amino acid tryptophan to indole-3-acetaldoxime by the action of cytochrome P450 monooxygenases belonging to family CYP79B2 and CYP79B3. Aldoximes is further catalyzed to form an uncharacterized intermediate under the action of cytochrome P450 monooxygenases (CYP83B1) which further converts to thiohydroximate by the inclusion of sulfur molecule by the activity of various enzymes namely glutathione S-transferases (GSTF9, GSTF10) and carbon-sulfur lyase (SUR1). Similar to aliphatic glucosinolate biosynthesis, glycosylation of thiohydroximate is catalyzed by uridine diphosphate glycosyltransferase 74(UGT74B1) followed by sulphation by sulfotransferases (SOT16) to form the core indole glucosinolate structure. The side chain modification takes place by processes such as hydroxylation. For instance, 4-hydroxyindole glucosinolate is produced by the action of CYP81F2. Further, the hydroxyindole glucosinolate molecules are converted to methoxyindole derivatives under the action of Indole Glucosinolate Methyltransferases 1 and 2 (IGMT1 and IGMT2) [28,29]. As far as the biosynthesis of aromatic glucosinolates is concerned very little knowledge is available and hence can be regarded as the neglected pathway. It is assumed that the chain elongation and core structure formation of aromatic glucosinolates is similar to indole glucosinolates however there is uncertainty whether the same genes control both the processes or there is a difference at the genetic level differentiating both the processes [30].
Glucosinolate degradation products
Myrosinase is a thio-glucosidase enzyme (EC3.2.1.147), present endogenously in the plants of Brassica species. The enzyme is present in idioblasts, also known as myrosin cells and is well separated from the glucosinolate molecules by compartmentalization within the plant tissues. Upon tissue damage, glucosinolates come in contact with the myrosinase enzyme, which is capable to hydrolyse the thioglucosidic bond in the glucosinolate structure, leading to the formation of biologically active degradation compounds. The degradation of glucosinolate is summarized in Figure 3 [31,32].
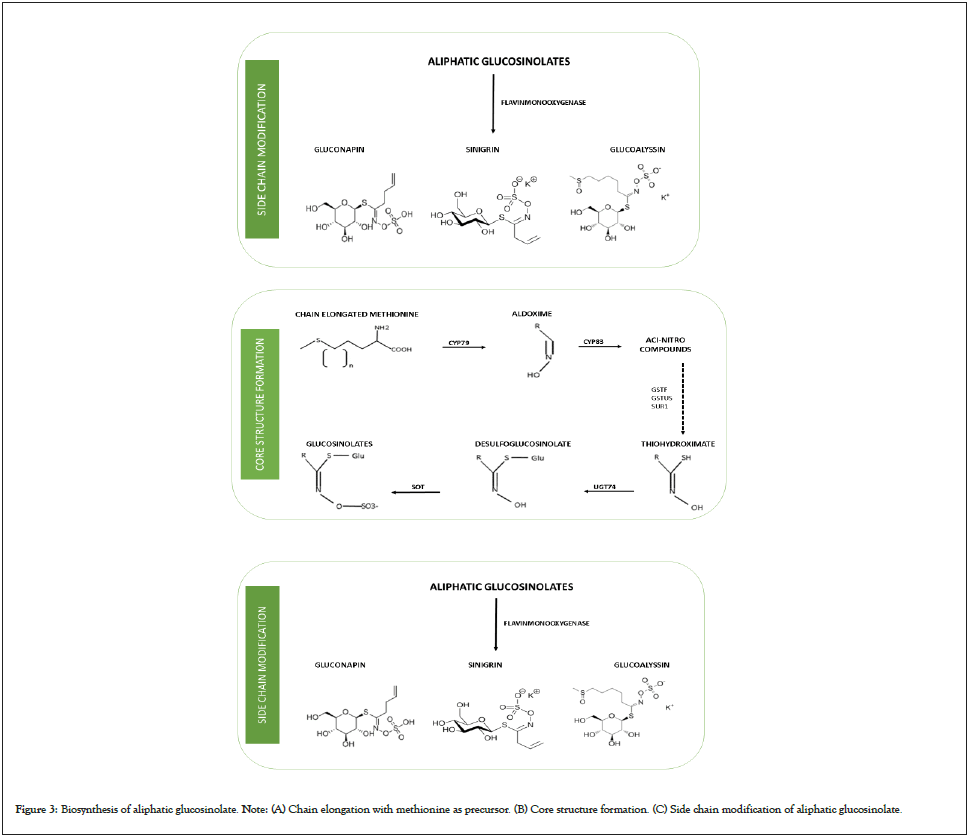
Figure 3: Biosynthesis of aliphatic glucosinolate. Note: (A) Chain elongation with methionine as precursor. (B) Core structure formation. (C) Side chain modification of aliphatic glucosinolate.
A neutral pH favors the formation of iso-thiocyanate, whereas the formation of oxazolidine-2-thione takes place by spontaneous cyclization of the isothiocyanate when the side chain of glucosinolate molecule is hydroxylated at carbon no 3. The hydrolysis of glucosinolates is directed towards the formation of epithionitriles and nitriles in presence of epithio specifier protein. Glucosinolates are even hydrolyzed to thiocyanates [33,34]. Apart from endogenous myrosinase enzyme, the gut microflora with myrosinase positive activity can assist the conversion of glucosinolates into physiologically active breakdown products. However, there may occur variation in the extent of hydrolysis of glucosinolates depending upon the difference in the gut microbiota of individuals. E. coli, Enterococcus faecalis, Enterococcus faecium, Lactobacillus agilis etc. which form a part of gut microbiota have been known to possess the capability to degrade the glucosinolates. Microbial strains from sources other than gut such as Aspergillus niger AKU3302, Aspergillus sp. NR46F13, Lactobacillus sp. LEM 220 have been reported to possess myrosinase activity [35,36]. The amount of glucosinolates in all the Brassica species varies significantly even between the plants of same species. The content of these sulfurous compounds is also tissue-specific. A great difference is observed in the glucosinolate content of different parts of a plant, with low to moderate quantities found in leaves and a significantly higher content found in seeds. Broccoli contains a good amount of glucosinolates predominantly glucoraphanin, ranging between 47 to 806 mg/100 g of fresh weight. The average glucosinolate content in Brussels sprouts falls in the range of 18 to 390 mg/100 g of fresh weight. Progoitrin, sinigrin, and glucoiberin have been identified as the main glucosinolates present in Brussel sprouts. A variation in glucosinolate content of cabbage has been noted based on the difference in variety. The glucosinolate content shows a significant variation in cabbage varieties with the highest content (148 mg/100 g of fresh weight) present in white cabbage followed by savoy cabbage and red cabbage with glucosinolate content of about 88 mg/100 g of fresh weight and 81 mg/100 g of fresh weight respectively. In white cauliflower, the total glucosinolate content ranges from 91 to 109 mg/100 g of fresh weight. The other varieties of cauliflower namely green cauliflower, Romanesco and purple cauliflower have a glucosinolate content of about 65 mg/100 g, 48 mg/100 g and 154 mg/100 g of fresh weight respectively. Chinese cabbage, one of the popular vegetables in China, Japan, and Korea contain gluconapin, glucobrassicanapin, and 4-methoxyglucobrassicin as the chief glucosinolate compounds with their content ranging between 49 to 246 mg/ 100 g of fresh weight. Aliphatic glucosinolates form the main glucosinolates of Kale. The average value of glucosinolates found in kale is 143 mg/100 g of fresh weight. The Ethopian kale has been found to be rich in sinigrin, which is the predominant glucosinolate present in it, accounting for 95% of the total content of glucosinolates that is about 43 mg/100 g of fresh weight. Knol khol, also termed as kohlrabi is known to possess a very high glucosinolate content with an average value of 295.5 mg/100 g of fresh weight. Brassica juncea, commonly known as mustard has a total glucosinolate content of about 121 mg/100 g of fresh weight. Various research investigations indicate that there is a difference in glucosinolate content in European and Asian cultivars with European cultivars characterized by lower levels of glucosinolates ranging between 26 to 155 mg/100 g of fresh weight as compared to Asian cultivars having glucosinolate content in the range of 208 to 354 mg/100 g of fresh weight.
The total glucosinolate content varies extensively among different varieties of radish ranging from 22 to 627 mg/100 g of fresh weight [37,38].
Health benefits associated with glucosinolates and their breakdown products PCR
Lower occurrences of numerous chronic diseases including cancer have been associated with intake of vegetable-rich diets by many clinical investigations. The cruciferous vegetables such as broccoli, kale, collard greens, cabbage etc. have been cultivated worldwide for ages and are being used widely either fresh, steamed, or cooked. Apart from being rich in nutritional components, these vegetables are known for their health- promoting secondary metabolites, of which glucosinolates and their breakdown products hold a prime position. In the recent past, extensive studies have been conducted on glucosinolates and their hydrolysis products. Based on the research data available, it is quite clear that the consumption of a diet containing these compounds could positively affect human health. Glucosinolates and their degradation products are known to be responsible for various physiological effects on health and many such effects are believed to be because of Brassica vegetables and these claims are based on in vitro, animal, human and epidemiological studies [39]. Different glucosinolates, their sources, breakdown products and pharmacological significance are summarized in Table 4. The hypothesis that a cruciferous vegetable-rich diet can reduce the risk of cancer has been supported by various epidemiological evidence. Consumption of cruciferous vegetables decreases the risk of development of bladder cancer by about 29% [40]. Isothiocyanate, the dominant breakdown product of glucosinolates has been linked to anti-carcinogenic activity and over the years there has been a growing interest in evaluating its effectiveness as a cancer-preventing agent. Two main mechanisms for the anti-carcinogenic effect have been proposed so far which include inactivation of the phase I enzyme cytochrome P450s binding with an isothiocyanate, as well as the induction of phase II enzymes and the induction of apoptosis which causes the deletion of genetically damaged cells and stopping the progression of the cell cycle. These two enzymes are known to play a key role in the metabolism, elimination, and detoxification of endogenous and exogenous carcinogens. The other mechanisms related to anti-cancer action are prevention of metastasis and angiogenesis and regulation of the epigenetic machinery [33,41] Sulforaphane, one of the intensively studied isothiocyanates derived from broccoli, is a potent inducer of cytoprotective enzymes in mammals. It is known for inhibiting the growth of many types of cancer cells including pancreatic cancer, colon cancer, leukemia, and prostate cancer, however, its use in cancer therapy is greatly restricted due to the short half-life [42,43]. Ascorbigen, one of the prime indole-derived, physiologically active compounds; is formed from glucobrassicin-a glucosinolate precursor, was first isolated from fresh cabbage juice [44]. It is found in various crops of Brassicaceae family. This Glucobrassicin-derived compound has been often linked to the anti-cancerous effect of diets rich in Brassica vegetables. Ascorbigen is also capable of inducing phase I and phase II enzymes that play a key role in xenobiotic detoxification [45]. Inflammation, a pervasive phenomenon, is a prime element of the defense system of the host that is triggered by innate immune receptors when a severe deviation of homeostasis occurs. It is characterized by edema, pain, redness and heat. However, dysregulation of inflammatory mechanisms has been linked to human cancers [46]. Gluconasturtiin, also termed phenyl thiocyanate exhibits remarkable chemopreventive potential along with having anti-oxidative and anti-inflammatory properties [47]. It has been observed that oral intake of this isothiocyanate tends to reduce the symptoms of ulcerative colitis, intestinal bleeding and inflammation of intestinal mucosa in mice [48]. Phenyl isothiocyanate has a unique ability to stop the progression of tumorigenesis along with preventing the initiation of the carcinogenic process [49]. The nuclear factor-kappa light chain enhancer of activated B cells (NF-kB), is a transcription factor that is primarily involved in the regulation of cellular behaviors. It is often associated with inflammation, diseases like arthritis and cancer. Gluconasturtiin is capable of inhibiting this transcription factor in colon cancer cells and macrophages [47]. Benzyl isothiocyanate has the potential to act as a chemo preventive agent in humans. It has been found to cause a dose-dependent reduction in the lipopolysaccharide- induced secretion of interleukin (IL)-1β, TNF-α and interleukin 6 [50]
| Glucosinolate | Chemical nature | Breakdown product | Dietary source | Pharmacological activity | Reference |
|---|---|---|---|---|---|
| Sinigrin | Aliphatic | Allyl iso thiocyanate | Broccoli, Brussels sprouts, cabbage, horseradish, mustard, radish | Anti-cancer, anti-inflammatory, antioxidant | (Mazumder et al.) [131] |
| Glucoalyssin | Aliphatic | Alyssin(5-(methylsulfinyl)-pentyl isothiocyanate) | Chinese cabbage | Anti-cancer | (Vig et al.) [132] |
| Glucoerucin | Aliphatic | Erucin(4-(methylthio)-3-butyl isothiocyanate) | Cabbage, Brussel sprouts, cauliflower, broccoli | Anti-cancer, antioxidant | (You et al.) [133] |
| Glucoraphasatin | Aliphatic | 4-Methylsulfanyl-3-butenyl isothiocyanate | White radish | Anti-mutagenic anti-cancer | (Suzuki et al.) [134] |
| Glucoraphanin | Aliphatic | Sulforaphane | Broccoli, Brussels sprouts, cabbage, turnip | Anticancer | (Tacer-Caba, 2019) [135] |
| Glucobrassicin | Indolyl | Indole-3-carbinol, Indole-3-acetonitrile | Cabbage, broccoli, cauliflower, Brussel sprouts,kale, kohl rabi | Anticancer | (Sayeed et al.) [136] |
| Gluconasturtiin | Aromatic | Phenethyl-isothiocyanate | Broccoli, turnip | Anticancer | (Hellin et al.) [137] |
Table 4: Glucosinolates and their breakdown products
Malignant melanoma, an aggressive, metastatic skin cancer is caused by prolonged exposure to UV radiations. Isothiocyanates have also been presented as anti-melanoma agents in various in vitro and in vivo research investigations. The glucosinolate breakdown compounds have been found to inhibit the growth of cells and induce programmed cell death (apoptosis). Moreover, isothiocyanates prevent metastasis by induction of signal transduction pathways, disruption of mitochondria and generation of oxidative stress. All these factors are known to be important mediators of cell growth and proliferation [51]. Butenyl thiocyanates, allyl isothiocyanates and related compounds exhibit dose-dependent radical scavenging ability and cytotoxic effect in cancer cells [52,53]. Many volatile isothiocyanates such as gluconapin, glucoerucin and glucoraphanin, released by glucosinolate breakdown show hepatoprotective effect. These compounds have an acetylcholinesterase inhibitory activity of about 53%. Isothiocyanates cross the blood-brain barrier, and hence are capable of exerting neuroprotective function. The literature available suggests a positive impact of isothiocyanates on various neurological diseases including Parkinson’s disease, Huntington’s disease, Alzheimer’s disease and ischemic brain injury, with the most promising activity displayed by phenyl isothiocyanate, having prime application in treating Alzheimer’s disease [54,55]. Sinigrin, a glucosinolate, commonly found in radish, cabbage, broccoli and Brussel sprouts, gives rise to allyl isothiocyanate upon enzymatic hydrolysis. It has been found to metabolize at a faster rate in the liver and the metabolites so formed affix with N-acetylcysteine and/or glutathione. These metabolites are also readily distributed to various body organs and tissues, and it has been noted that they exert an anti-obesity effect in rats. Ally isothiocyanate is also known to possess anti-diabetic properties [56]. Erucin, is formed by enzymatic hydrolysis of Glucoerucin which is a precursor for eurcin or by in vivo reduction of sulforaphane Erucin has been investigated for its anti-inflammatory effect. It has been found that this compound significantly reduces the expression of pro-inflammatory cytokines such as Tumour Necrosis Factor- alpha (TNF alpha), Interleukin 1 beta (IL1-β) or leukocytic pyrogen and Interleukin 12 (IL-12) in the human monocytic cell line (THP-1) which is derived from a patient suffering from acute monocytic leukemia [57-59]. Type 2 diabetes, is one of the most prevalent endocrine and metabolic disorder. The consumption of foods with nutraceutical potential is by far the newest approach in managing this disorder. Many in vivo studies and clinical trials suggest that glucosinolates, particularly their breakdown products, such as sulforaphane can be an effective adjunct in the management and prevention of diabetes in long run [60].
Pre-harvest factors affecting glucosinolate content
Various pre-harvest factors have been known to have an impact on the synthesis, accumulation and profile of glucosinolates.
Stages of development at harvesting and plant parts: The developmental phase can influence the concentration of phytochemicals in Brassica especially glucosinolates. In general, it has been noted that the total glucosinolate content varies through different growth phases and an increasing trend has been observed in glucosinolate content from vegetative to reproductive and maturity stages [61]. The elevated levels of glucosinolates were found in flower buds and leaves of kale harvested at the recommended consumption stage after 180 days of sowing. Similarly, broccoli heads showed a high content of glucosinolate namely glucoraphanin 180 days after sowing which significantly decreased during the flowering period [62,63]. There is a no table difference in glucosinolate content based on the plant part, with seeds having the highest concentration of glucosinolates followed by siliques, inflorescence, leaves, roots, stems and petioles [64]. A research study indicated that Turnip tops have a glucosinolate content of 26.02 μmol/g on a dry weight basis which is higher than turnip greens having glucosinolate content of 17.78 μmol/g on a dry weight basis [65].
Environmental factors: Many environmental factors such as seasonal variation, light exposure, temperature, water availability, etc. have been reported to influence the quality, nutritional and phytochemical content of Brassica crops. Numerous research studies are favoring the fact that spring season crops have a higher glucosinolate content owing to various favorable conditions during the vegetative period particularly intermediate temperatures, high light intensity, longer days and dry conditions. Autumn and winter season plants that are grown at a lower temperature, lower light intensity, shorter days, and higher water availability, tend to have the lowest concentrations of glucosinolates and other important phytochemicals [61,66]. Differential activation of enzymes linked with glucosinolate biosynthesis by light and temperature is a possible reason for the higher content of 4-methylsulfonylbutyl-GL and 4-methylsulfonylbutyl isothiocyanate in Broccoli sprouts of cultivar Youxiu at 25°C while as comparatively lower levels have been reported in broccoli sprouts grown at lower (20°C) and higher temperatures (30°C) [67]. It has been reported that a temperature of 40°C maintained for 4 hours for 5 days in a row resulted in an increased concentration of glucosinolates in Brassica napus [68]. Water stress has also been noted to increase the glucosinolate content [32]. Brassica napus and Brassica rapa when subjected to water stress showed a significant increase in glucosinolate content of seeds that is 35.0 mmol/g as compared to seeds from non-stressed plants having glucosinolate content of 18.2 mmol/g [69].
Agricultural practices: A deep-rooted qualitative, as well as the quantitative effect on glucosinolate content in Brassica species due to agricultural management, is well known. Many researchers believe that the concentration of secondary metabolites particularly glucosinolates is higher in crops grown under the organic farming system as compared to those cultivated under conventional system and this outcome may be attributed to the much greater stress conditions to which the crop is subjected under organic system leading to increased production of secondary metabolites as a part of plant defense mechanism. A significantly higher concentration of 3-indolylmethyl-glucosinolate has been noted in broccoli grown under organic conditions as compared to the counterparts grown conventionally. A similar outcome has been noted in tronchuda cabbage [61,70,71]. However, some research reports are contradicting such claims. A higher concentration of 3-indolylmethyl-glucosinolate and 1-methoxy- 3-indolylmethyl-glucosinolate has been observed in broccoli grown under the conventional system when compared to those grown under the organic system [71]. The use of fertilizer during growth is known to modify the balance of the mineral content of soil particularly the balance between nitrogen and sulfur thereby seemingly changing the concentration and profile of various secondary metabolites including glucosinolates [72]. An experiment conducted by Park, et al. suggests a correlation between sulfur and an increase in glucosinolate content [73]. As per the experiment, enhancement of soil with sulfur during the cultivation of crops may lead to increased levels of glucosinolates since this element forms a prime part of the glucosinolate core structure. A significant increase in the level of total glucosinolate content of Brassica rapa and glucoraphanin, sinigrin, gluconapin and Progoitrin in Brassica juncea has been observed with an increase in the sulfur content of soil [74]. In general, the use of nitrogen-based fertilizers has been linked with decreased levels of glucosinolates in plants. However, the effect of nitrogen varies according to the type of glucosinolate. It has been noted that excessive use of nitrogen leads to an increase in the production of progoitrin but at the same time sinigrin concentration decrease in Brassica napus [75]. An optimal balance between sulfur and nitrogen is necessary for the proper growth and development of the plant along with the production of glucosinolates. It has been indicated in one of the research studies that the use of high levels of both sulfur and nitrogen can lead to the efficient working of various key enzymes associated with assimilation of sulfur there by resulting in higher levels of glucosinolates in Brassica napus [76]. The use of NO3 and NH4 in combination with a combined ratio of 1:1 has been established as an optimal fertilizer combination to achieve a good concentration of glucosinolates in Brassica plants as noted in the case of Chinese kale [77]. The type of fertilizer used can impact the mineral composition of soil which in turn influences the mineral composition of plants. The application of nitrogen fertilizers is negatively correlated to mineral profile especially potassium and calcium while as sulphur-based fertilizers have been positively correlated with manganese and zinc. Selenium in the form of sodium selenate has been associated with an increase in glucosinolates particularly sulforaphane. However, this effect is dose-dependent, where selenium used above certain level tend to decrease overall content of glucosinolates [61,78,79]. In-field application of sodium selenite along with sodium chloride showed increased levels of isothiocyanates in kale however it also resulted in a decrease in levels of glucoraphanin and sinigrin [80]. Apart from chemical/ synthetic fertilizers, manure obtained from different sources has been used to supplement the soil for the cultivation of Brassica crops. A higher concentration of glucosinolates (1287 mg/g) has been observed in Brassica juncea grown on soil supplemented with sewage sludge. A slightly lower level of glucosinolates (929 mg/g) has been recorded in crops grown on soil with added chicken manure [81]. Various compounds which prove to be beneficial for the production of glucosinolates and other bioactive comparts apart from traditionally used chemical fertilizers have been proposed recently. Plant hormones namely Jasmonic acid, Salicylic Acid and Abscisic acid have been closely related to the regulation of glucosinolate content in crops. It has been noted that treatment with Jasmonic acid and methyl jasmonate leads to 20 times increase in the concentration of Glucobrassicin in B. napus, B. rapa and B. juncea leaves and seeds. The concentration of indole glucosinolates decreased in Brassica napus on treatment with abscisic acid [72,82,83].
Effect of processing on glucosinolate content
Before consumption, the Brassica vegetables are subjected to several processing operations at industrial and domestic levels which include thawing, boiling, washing, cutting, chopping, blanching, freezing, microwaving, etc. to enhance their palatability, digestibility and sensory attributes. However, these processes exert stress on the cellular structure of plants and may influence stability, and the levels of various phytochemicals including glucosinolates, ascorbic acid and polyphenols. The processing methods are varied particularly at the domestic level and greatly depend on the type of the vegetables, the desired quality features of intended end products, and the local customs and traditions. On an industrial/commercial scale, the preparation and processing methods are standardized [84,85]. It has been noted that the processing techniques usually result in the degradation and depletion of the biologically active, natural components. However, in some cases, it has been seen that there occurs no change in the phytochemical content upon processing. Moreover, some processing methods have also been linked to the formation of certain novel compounds that exhibit biological activity [86, 87].
Glucosinolates are affected during food preparation and processing. The degree of disruption and changes in the content of glucosinolates depends on the type of vegetable undergoing processing and the method of processing. The majority of the processing methods used for Brassica vegetables are thermal in nature and hence involve the application of heat [88]. A model proposed to explain the mechanism of glucosinolate degradation during the processing of Brassica vegetables involves many steps that take place either in a sequential manner or simultaneously [89]. The breakdown of these sulfurous compounds can take place via the following steps:
• Rupturing of cells and cellular compartments.
• Dissemination of cellular components through the ruptured tissue.
• Enzymatic hydrolysis of the glucosinolate molecules due to tissue breakdown which leads to contact between myrosinase enzyme and the glucosinolate molecules.
• Thermal degradation of glucosinolates and their breakdown products.
• Inactivation of myrosinase enzyme due to high temperatures as well as loss of various enzymatic co-factors, namely ascorbic acid, Fe2+, epithio specifier protein, or thiocyanate- forming protein.
• Leaching of glucosinolates and breakdown products into boiling water after rupturing of the cell.
Boiling and blanching: Boiling and blanching are the most commonly employed processing methods applied on brassica vegetables. In case of boiling, the vegetables are immersed in boiling water at a temperature of 100°C. The heating of vegetables results in the rupturing of cellular compartments and tissues resulting in leaching out of myrosinase enzyme and glucosinolates from the vegetable tissue into the cooking water. This leads to the enzymatic hydrolysis of the glucosinolates in the damaged tissue as well as the surrounding water used for boiling. Glucosinolate molecules are labile to heat and hence undergo thermal/non-enzymatic degradation at boiling temperatures [90,91]. The degree of loss of glucosinolates due to heat depends on their chemical structure, with indole glucosinolate being more sensitive to heat than the aliphatic ones [85]. Blanching, which involves short-term heating, has been associated with an increase of isothiocyanate content probably due to the inactivation of epithio specifier protein, which is one of the prime enzymatic co-factor [92]. Kapusta-Duch, et al. reported a significant decrease in the level of glucosinolate and its breakdown compounds of green and purple cauliflower and rutabaga upon boiling for 15 minutes [93]. The boiling was found to induce glucosinolate losses ranging from 6.6% in rutabaga to about 69.0% in both green and purple cauliflowers.
A small yet statistically significant loss was reported by Rungapamestry, et al. in the total glucosinolate content of Broccoli after boiling and this meager loss has been linked to the shorter boiling period used [94]. A higher degree of loss of glucosinolates has been noted in Broccoli when boiled at high pressure [95]. Lafarga, et al. suggested that blanching at 95°C for 3 minutes did not have a significant deterioration impact on glucosinolate content as about 100% of aliphatic and 98% of indole glucosinolates were retained after the process [90]. Few Brassica vegetables to boiling for about 30 minutes and observed that there occurred a decrease in glucosinolate content by 58%– 77% from base values with 77% loss recorded in broccoli, 75% in cauliflower, followed by green cabbage and Brussels sprouts with a loss percentage of 65% and 58% respectively [96]. The glucosinolate content of blanched Brassica vegetables showed a significant decrease, with losses ranging from 2.7% in green cauliflower, 13.0% in white cauliflower, to 21.1% in curly kale and 30.0% in Brussels sprouts and broccoli [97]. The research report also suggested a drastic loss of glucosinolates in vegetables after boiling, ranging between 35.3% in white cauliflower to 72.4% in curly kale [97].
Steaming: Steaming is considered the most beneficial method for retaining the valuable, biologically active components wherein steam produced by vaporizing water under boiling conditions is applied to vegetables [32,85]. In the case of Brassica vegetables, the process of steaming is noted to have a minimal impact on the glucosinolate content owing to the least tissue rupturing, lower rate of myrosinase inactivation and a considerably low heat/ thermal damage to the glucosinolates [91]. The literature available suggests that steaming has minimal impact on the glucosinolate content; it either decreases the number of glucosinolates slightly or results in a slight increase in the glucosinolates in Brassica vegetables. Rungapamestry, et al. reported no significant loss in the glucosinolate content in cabbage when it was steamed for 7 minutes [98]. Conaway, et al. and Vallejo, et al. also suggested that steaming does not affect the glucosinolate content of cabbage and broccoli when compared to the glucosinolate content of fresh counterparts [95,99]. Steaming for 20 minutes resulted in no significant decrease in the glucosinolate content of brassica vegetables namely broccoli, green cabbage, cauliflower and Brussels sprout [96]. No significant amount of glucosinolate breakdown products mainly sulforaphane was detected after steaming broccoli. The glucosinolate content of red cabbage was found to suffer a 6% loss upon steaming and the overall glucosinolate content of broccoli showed an increase of 12% when subjected to steam for 15 minutes [83,100].
Frying: Stir-frying, a cooking method considered to be more favorable than normal frying, has its origin in Asia and involves frying food using a small quantity of oil. Due to culinary expansion in the world, this method is gaining popularity globally. It has been reported that glucosinolates were retained in Chinese cabbage when stir-fried even at a high temperature of about 250°C. It is believed that glucosinolate content is not affected due to a quick inactivation of glucosinolate hydrolyzing enzyme-myrosinase. Moreover, the absence of cooking water rules out the process of leaching out of these sulfurous molecules [39]. Miglio, et al. reported that deep-frying harmed the glucosinolate content of broccoli due to thermal degradation [101]. Deep frying was reported to cause an 84% reduction in the glucosinolate content of broccoli.
Microwave: Microwave processing has gained a lot of attention in recent years in the food sector due to the notable reduction in cooking time and overall energy consumption. Microwaves are electromagnetic waves with a frequency range of 300 MHz to 300 GHz. The field generated by these waves is alternating in nature, thereby forcing the polar molecules to change their orientation continuously under the electric field direction. The suitability of food materials to be processed by microwaves has been linked with the dielectric properties possessed by food materials [102]. The electric field component of the microwave induces the rotation of dipoles in foods and the heat is generated by the friction of molecules [103]. Various mechanisms underlying in determining the glucosinolate loss such as cell lysis and enzyme inactivation, occur during microwave processing. The extent of changes taking place in glucosinolate content greatly depends on the processing time, wherein it has been observed that longer treatment periods result in increased tissue damage, cell rupturing and thermal degradation of glucosinolate molecules [32]. The activity of myrosinase enzyme is known to increase at moderate temperatures of 60°C, however at higher temperatures, there occurs a rapid inactivation of this enzyme. The leaching out of glucosinolate molecules into the surrounding water used for cooking is only possible in microwave processing when a fair amount of water is added to the vegetables before subjecting to microwave cooking [95,104,105]. There are controversial reports available in the literature regarding the impact of microwave processing on the total glucosinolate content of Brassica vegetables. Many researchers consider microwave processing as a novel method having minimal effect on the bioactive components, especially health-promoting glucosinolates. However, there exist few research works that have reported a significant loss of glucosinolate content upon microwaving. A slight increase in the glucosinolate content of red cabbage when microwaved at three different output powers and time durations [105]. Contrary to this finding Rungapamestry, et al. noted that the glucosinolate content of cabbage decreased slightly when microwaved [98].
Fermentation: Fermentation is one of the oldest and simplest processing techniques employed worldwide to enhance the nutritional and sensory quality of fruits and vegetables apart from extending the shelf life [106]. Vegetables including those belonging to the genus Brassica undergo lactic acid fermentation, wherein fermentation is carried out by the lactic acid bacteria that grow either spontaneously or are added in the form of starter cultures [107]. It has been reported that conventional fermentation of cabbage such as the production of sauerkraut, leads to complete degradation of glucosinolates. Fermented cabbage contained no glucosinolates [108]. Sarvan, et al. suggested that thermal pre-treatment in the form of blanching before fermentation by a probiotic strain LMG P22043 retained 35% of the original total glucosinolate content in cabbage [109]. The blanched, fermented cabbage contained 27.2 ± 2.3 μmol/100 g of glucosinolate after 71 hours of Lactobacillus paracasei fermentation. Traditionally fermented mustard (Brassica juncea) leaves known as sayur asin, are commonly consumed in Indonesia. The leaves of Brassica juncea, are known to be rich in aliphatic glucosinolates particularly sinigrin. It has been known that fermentation can significantly reduce the glucosinolate content owing to the activity of myrosinase enzyme during the process. Various pre-treatments were evaluated to note their efficiency in retaining the glucosinolate content in fermented product-Sayur asin. Withering of leaves by various methods was done to reduce the myrosinase activity. Withering by oven at 35°C for 2.5 h and by microwave at 180 W for 4.5 min were reported to reduce the enzymatic activity by about 84 and 74%, respectively. However, these treatments were not sufficient to save the sinigrin content as it was not detected after 24 hours of fermentation. Microwave withering at 900 W for 2 minutes proved to be an effective treatment as myrosinase enzyme was completely inactivated, and sinigrin at a concentration of 11.4 μmol/10 g dry matter was noted in sayur asin after 7 days of fermentation [91]. Palani, et al. investigated the influence of fermentation on glucosinolates and their breakdown products [110]. Cabbage fermented at 20°C and kept under storage at 4°C was evaluated for glucosinolate content. It was noted that the glucosinolate content showed a dramatic decrease between day 2 to day 5 of fermentation, and by day 7 no traces of glucosinolates were found in the fermented product. However, various biologically active degradation products were detected namely ascorbigen and indole-3-carbinol. Investigated the effect of lactic acid fermentation on glucosinolate content particularly the glucoiberin, progoitrin and glucoraphanin of autoclaved broccoli puree [111]. The total concentrations of glucosinolates showed an increase upon fermentation, ranging from 55 μg/g to 359 μg/g. An increase in glucoraphanin concentration from minute concentrations in the autoclaved broccoli to 29-237 μg/g after fermentation was noted, thereby presenting fermentation as a promising process to elevate the glucosinolate content. Arount 12 glucosinolate breakdown products have been identified in fermented cabbage. Ciska, et al. reported sinigrin as one of the abundant glucosinolate present in shrerdded cabbage which was hydrolysed to allyl isothiocyanate during the fermentation process. It has also been reported that other glucosinolates were hydrolysed to isothiocyanates and cyanides [112].
Other processing techniques
The effects of other processing methods on the glucosinolate content of Brassica vegetables have also been studied. Various novel technologies for the processing of vegetables, including high-pressure processing, pulsed electric field, UV radiations, etc have been evaluated to check their potential and suitability to be used as alternatives for thermal processing. It has been noted that the glucosinolate content of vegetables can be retained upon freezing and subsequent frozen storage. Rungapamestry, et al. reported that freezing blanched broccoli at -18°C had no significant change in the glucosinolate content [94]. Substantiating the earlier studies Volden, et al. reported no change in the glucosinolate content of cauliflower when stored under frozen conditions for 12 months [85]. Storage of broccoli, Brussels sprouts, cauliflower, and green cabbage under frozen conditions for two months without prior blanching resulted in significant loss of glucosinolates probably due to the freeze-thaw damage of plant cells leading to release of myrosinase and glucosinolates [96]. The effect of high-pressure processing on glucosinolates has also been of research interest in the recent past. High-pressure processing, a non-thermal process, has been identified as one of the best alternative technique for processing and sterilizing foods by applying high pressure ranging between 100 MPa to 1000 MPa for short time durations, which leads to inactivation of many indigenous food enzymes, pathogenic and spoilage microorganisms along with maintaining the content of bioactive compounds [113]. Many research investigations have been carried out to evaluate the impact of HPP on glucosinolates. Westphal, et al. reported about 85% conversion of glucosinolates into isothiocyanates possibly due to the inactivation of ESP after high-pressure treatment of 600 MPa for 3 minutes on broccoli sprouts [114]. Alvarez-Jubete, et al. reported higher concentrations of isothiocyanates after subjecting white cabbage to High- pressure processing at 600 MPa when compared to blanching [115]. The use of Ultraviolet (UV) radiations has grown as a promising alternative to decontaminate foods and contact surfaces [116]. The use of UV rays has successfully modified the activity of enzymes indigenous to cole vegetables to enhance the production of bioactive compounds namely flavonoids and ascorbic acid. However, a very meager amount of related data is available regarding glucosinolates and their breakdown components [117,118].
The majority of the research data available in this context is focused on broccoli and hence knowledge regarding the effect of UV radiations on other cruciferous vegetables is not available. Formica-Oliveira, et al., observed that single or combined UV-B and UV-C at an intensity of 5-15 kJ/m2 and 9 kJ/-m2 respectively could increase the concertation of glucosinolates in broccoli by-products after a 3-day storage period [119]. Treatment of broccoli with UV radiation (2.2, 8.8, and 16.4 kJ/m2/day) during the vegetative period can also result in a significant increase in glucosinolates content [116]. Similar observations have been made by who reported an increase in the content of glucosinolates in broccoli sprouts after pre-harvest UV-B radiation at 0.3-1.0 kJ/m2/ day [120]. Moreira-Rodríguez, et al. observed maximum glucosinolate accumulation in broccoli sprouts 24 hours after UVB treatment with an intensity of 3.34 W/m2 [121-137].
Genus Brassica, a vast genus belonging to the family Brassicaceae encompasses about 3709 species. The regular consumption of a diet rich in Brassica vegetables has been linked to various positive biological and pharmacological activities owing to the rich nutritional and phytochemical composition of these vegetables particularly the presence of biologically active molecules known as glucosinolates and their breakdown products. A profound amount of literature is available in favor of the anti-cancer property of these compounds. Processing, which aims at improving the bioavailability of nutrients, palatability and shelf-life of the foods has been known to alter the glucosinolate content of Brassica vegetables. However, the mechanism behind the effect of different processing conditions on glucosinolate content and its degradation rate is not fully understood and hence there is a need for mechanistic studies to reveal and understand the breakdown mechanisms of glucosinolate molecules. Furthermore, studies regarding various region and culture-specific preparation and processing techniques will aid in enhancing the knowledge and understanding related to glucosinolates and their behavior under different processing conditions.
The authors declare there are no financial or competing interests.
The authors are thankful for financial support by National Mission for Himalayan Studies (NMHS) under grant number GBPI/NMHS-2019-20/SG, dated 30/09/2019.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Rizwan D, Sidiq M, Masoodi FA. (2023) Glucosinolates from Brassica Vegetables: Their Potential Health Benefits and Effect of Pre-Harvest and Processing Factors on their Content. Med Aromat Plant.12:458.
Received: 18-Oct-2023, Manuscript No. MAP-23-27623; Editor assigned: 20-Oct-2023, Pre QC No. MAP-23-27623 (PQ); Reviewed: 03-Nov-2023, QC No. MAP-23-27623; Revised: 10-Nov-2023, Manuscript No. MAP-23-27623 (R); Published: 17-Nov-2023 , DOI: 10.35248/2167-0412.23.12.458
Copyright: ©2023 Rizwan D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.