Forest Research: Open Access
Open Access
ISSN: 2168-9776
ISSN: 2168-9776
Research Article - (2013) Volume 2, Issue 1
The effect of plantation forests on the global carbon balance is controversially discussed in recent times. As soil respiration is a decisive component in the carbon exchange between terrestrial ecosystems and atmosphere, effects of forest management measures (e.g. thinning) in the context of driving parameters of soil CO2 efflux is a key issue in optimizing carbon friendly land management. In the present study, we report the effects of thinning, soil temperature and soil moisture, and biotic parameters on soil CO2 efflux rate. Soil CO2 efflux was measured by using an Infrared Gas Analyzer. We selected thinned and un-thinned stands within six years old Cupressus lusitanica plantation forest. Soil respiration rate ranged from 1.47 to 6.92 µmol m-2s-1 (thinned) and 1.31 to 5.20 µmol m-2s-1 (control stand).
Generally higher soil respiration rates were measured during wet than in dry season. Seasonal variability of soil CO2 efflux was significantly (p<0.05) correlated with soil moisture, but poorly correlated with soil temperature. Soil respiration increased with increasing soil moisture and reached maximum at 31% but after this threshold it start to decline. In general, soil CO2 efflux rate in the first and second year after thinning was 24% and 14% higher in the thinned stand. Increased soil temperature at the thinned stand contributed minor to the larger soil CO2 efflux, the more important reason appeared to be the trees’ direct response. Higher fine root production together with larger microbial concentrations representing different groups infers a higher autotrophic respiration by roots and associated mycorrhizal fungi as well as by heterotrophic respiration. Despite the higher CO2 losses with soil respiration, the
organic C and total N concentrations in soil rather tended to increase, indicating higher organic matter input to soil at the thinned stand.
Keywords: Cupressus lusitanica, Soil CO2 efflux, Soil moisture, Soil temperature, Thinning
Recent atmospheric studies have suggested a substantial terrestrial sink for CO2 could be attributable to the African continent [1,2]. However, there is still great uncertainty of the magnitude and variability of the African carbon stocks and fluxes [3]. The African ecosystems contribute 20% of global net primary production, along with the same proportion on global forest degradation and deforestation induced CO2 emission [4], of which 0.24 Pg C yr-1 for sub-Saharan Africa [5].
One component that needs to be addressed in the terrestrial carbon budget is the efflux of CO2 from the soil surface to atmosphere as being ultimately attributable to the ecosystem biological activity [6]. Contributing more than 50% to the ecosystem respiration across variety of biomes [7,8], soil respiration determines the ecosystem carbon balance and thus an ecosystem sink or source activity [9]. Simulations of the soil CO2 efflux for biogeochemical models are one of the main challenges due to high temporal variability and spatial heterogeneity of soil respiration at all scales with respect to environmental conditions and long-term site disturbance history. Soil temperature and soil moisture has been recognized to be the two most influential abiotic parameters leading to seasonal and diurnal variations of soil respiration in different ecosystems [10-12].
One of the economically viable strategies for sequestering atmospheric carbon and mitigation the climate change scenario in tropics could be development of plantations on suitable land areas [13]. The Global Forest Resource Assessment reported that in 2010 planted forests worldwide accounted 264 million hectare, 7.2 million hectare of which were located in sub-Saharan Africa. Among other African countries, Ethiopia has one of the longest forest plantation histories [14] with the current total plantation forests area of 511 thousand hectare [15]. Forest plantations in Ethiopia are mainly represented by monocultures of fast growing exotic tree species mainly Eucalyptus sp. and Cupressus lusitanica [16]. Productivity of plantations needs to be high to meet increasing demand of wood products and to enhance stand quality and ability of effective carbon sequestration in various compartments of a forest ecosystem [17].
Forest management practices may determine carbon exchange between the atmosphere and the forest [18]. One measure to increase the formation in tree biomass, mainly stems, is thinning. This practice affects physiological and hence growth responses of the remaining trees and micro-climatic changes within the modified stand [19], influencing the environmental condition beneath the canopy, including the soil microclimate, and root dynamics [20]. The aboveground productivity and the carbon transfer to soil via litter fall and rhizo deposition are decisive for carbon availability for microbial decomposition processes in soil. Hence, it is likely that changes in aboveground productivity will have consequences for belowground process and vice versa [21]. All these changes may be reflected in the soil respiration. Most research on thinning treatment on plantation forests, including Ethiopia [22] has been done in order to identify management guidelines to produce optimal stand growth [23]. Few studies have now emerged that focused on the carbon implications of thinning while targeting volume production [24]. As principally soil CO2 flux studies in African intact and plantation forests are scarce [25-28], to our knowledge no study investigated the impact of thinning on soil respiration in east African plantation forests.
The Munessa forest is one of the largest plantation forest in Ethiopia established in the 1970’s. It covers about 6973 ha of which Cupressus lusitanica (Mill.) is the dominant planted tree species that account for 60% of the total plantation area [29]. The objectives of this study were (i) to compare the effects of thinning treatment on soil CO2 efflux rate between intact and thinned stands and (ii) to compare indicators for soil microbial biomass between intact and thinned stand. We hypothesized that thinning treatment would increase soil CO2 efflux rate by stimulating the productivity of the remaining trees [30] and changing the soil microclimate conditions and as a result of improved growing conditions of remaining trees and change in soil microclimate, soil microbial biomass will be larger under thinned stand.
Study site and silvicultural treatment
The study was conducted in 6-years-old Cupressus lusitanica stand established in June 2003 located at the Munessa forest (7°25’ 44”N and 38°51’ 05”E), West Arsi zone, Oromia regional state, Ethiopia. The slope is gentle and the elevation at the study site is 2126 m above sea level. The soil texture is predominately a slightly acidic and nutrient rich clay-loam that was evolved from volcanic parent material [31], and was classified as Mollic Nitisols according to the WRB system [32]. The chemical and physical properties of the surface soil of the experimental plots are presented in Table 1.
| Stand | Soil depth | Sand* | Silt* | Clay* | pH§ | CEC¶ | BS** |
| –––– g kg-1 ––– | mmol(+) kg-1 | % | |||||
| Thinned | 0-10 cm | 210 ± 15 | 395 ± 62 | 395 ± 67 | 6.0 ± 0.5 | 638 ± 92 | 100 |
| 10-25 cm | 189 ± 8 | 371 ± 65 | 440 ± 70 | 5.7 ± 0.6 | 449 ± 69 | 100 | |
| Control | 0-10 cm | 203 ± 20 | 376 ± 20 | 421 ± 20 | 6.3 ± 0.5 | 568 ± 24 | 100 |
| 10-25 cm | 190 ± 26 | 329 ± 7 | 481 ± 27 | 5.7 ± 0.7 | 447 ± 74 | 100 | |
*measured by the pipette method (33); § analyzed potentiometrically in 1 M KCl [1:2.5 (m/v)]; ¶ cation exchange capacity determined with the BaCl2 compulsive exchange method (34); ** base saturation. Values are mean and standard deviation of three replicated samples
Table 1: Basic characteristic of the soils under each stand.
The study site is characterized by a bimodal rainfall pattern with a minor rainy season occurring from March to May and a major rainy season from July to November [33-35]. Own meteorological records since 2001 show that 80% of the annual precipitation fell in the major rain season, and no clear indication of a minor rainy season is given. Also the precipitation pattern during the observation time showed pronounced seasonality with a wet period from June to October 2009 and from February to October 2010 showing a monthly precipitation of up to 200 mm (Figure 1). In contrast, November 2009 to January 2010 and the last six months were characterized by dry conditions. Minima of air temperature occurred at f the transition of the rainy season to the dry season with an average monthly temperature of c. 14°C and increased towards the transition of the dry season to the rainy season peaking in an average monthly temperature of c. 19°C.
Figure 1: Seasonal patterns of air and soil temperatures (a), precipitation and soil moisture (b), and soil CO2 efflux rate (c). Each data point for soil respiration, soil temperature and soil moisture is a mean of twenty measurements. Error bars indicate standard deviation. For the air temperature the monthly average temperatures measured by the Kuke weather station are presented. Data gaps are due to rain events or instrument failure. Periods with light grey background indicate wet periods as calculated by the approaches of Gibbs and Maher [52].
For the present study, we worked on two fenced stands (40m×40m) located 20m apart from each other. The experimental stands were selected by a silvicultural research team with the objective to investigate the influence of thinning operation on the growth performance of potential crop trees of C. lusitanica. The stand was initially planted at spacing of 2.5m×2.5m. In January 2008, selective thinning has been done in one of the fenced areas by removing the competitors trees next to trees assigned as potential crop. No thinning operation was carried out on the other fenced area and was considered as a control. After thinning the number of trees left in the thinned stand was 98 as compared to 144 in the control stand.
Soil respiration measurement
Before the start of the field campaign, within each stand sixteen randomly spots were selected in order to estimate the number of sampling points required for soil CO2 efflux measurement. At each selected spot, circular PVC soil collars (20 cm in diameter and 5 cm long) were installed about one year and six months of lag time after the management impact. An insertion depth of about 1-2 cm into was chosen for soil CO2 efflux measurement to minimize severing of roots and the mycorrhiza. Finally, the outer wall of the collars was sealed with fine sand to avoid leaking of the chamber-soil system.
The number of individual flux measurements needed for various degrees of precision at various confidence levels was computed following probability equation described by Snedecor and Cochran [36]:
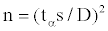 (1)
(1)
where tα is Student’s t with degrees of freedom at the 0.05 probability level, s is the standard deviation with values obtained at this study, and D is the specified error limit. The result in Table 2 shows the required number of measurement points in each of the stands under different degrees of precision. Clearly, large numbers of flux measurements are ideal but we learnt from the 16 collar measurements before the start of the sampling campaign, it was feasible to restrict our measurements on 20 collars per stand. This decision led us our soil CO2 efflux estimation to fall within an interval of 20% of the population mean at 95% confidence interval (Table 2).
| Interval about the full population mean (%) | 99% confidence (α=0.01) | 95% confidence (α=0.01) | 90% confidence (α=0.01) | 80% confidence (α=0.01) | ||||
| Thinned | Control | Thinned | Control | Thinned | Control | Thinned | Control | |
| ± 10 | 31.95 | 34.10 | 24.27 | 25.91 | 20.37 | 21.74 | 15.85 | 16.92 |
| ± 20 | 26.85 | 28.65 | 20.40 | 21.77 | 17.12 | 18.27 | 13.32 | 14.22 |
| ± 30 | 22.88 | 24.41 | 17.38 | 18.55 | 14.59 | 15.57 | 11.35 | 12.11 |
| ± 40 | 19.73 | 21.05 | 14.99 | 15.99 | 12.58 | 13.42 | 9.79 | 10.44 |
| ± 50 | 17.18 | 18.34 | 13.05 | 13.93 | 10.96 | 11.69 | 8.52 | 9.10 |
Mean and standard deviation of soil CO2 efflux on the measurement day was 5.34 ± 1.1 and 5.10 ± 0.8 μmol m-2s-1 for thinned and control stand respectively
Table 2: Number of sampling point required within a precision of ± 10 to ± 50% of the full population mean and at different confidence interval between 80-99% based on 16 measurements per stand in May 2009.
At each stand, we installed the 20 collars under the canopy of four individual randomly selected trees at a distance of about 0.7 to 1.3 m from the bole. This design was chosen because previous studies had shown higher variability and higher soil respiration rates were observed in collars established at close vicinity to tree stems [37,38]. A sampling design of installing collars close to the vicinity to tree stems gives more reliable estimates of fluxes from a given stand [39]. Olsthoorn et al. [40] has shown that such sampling design was also more error free in the assessment of fine root density. Soil collars were left in place throughout the measurement period. Herbaceous understory vegetation was avoided during collar set-up. However, when, in the following two year’s period, any vegetation grew inside the collars, it was clipped back.
Soil CO2 efflux was measured on a weekly basis from May 2009 through May 2011 with the exception of November and December 2009 due to instrument failure. Instantaneous soil respiration was measured using a LI-8100-103 soil survey chamber (LI-COR, Lincoln, NE, USA) connected to an Infrared Gas Analyzer Li-8100 (LI-COR, Lincoln, NE, USA). Soil temperature (°C) at a depth of 0.1 m was recorded adjacent to each collar simultaneously with soil CO2 efflux measurements using a thermocouple probe (Li-8100-201) connected to the Li-8100. The volumetric soil water content at 0.06 m depth was also measured with a handheld theta probe (ML2, Delta-T Device Ltd, Cambridge, UK) at three replicates around the collars immediately following each soil CO2 efflux measurement. For each of the stands mean CO2 efflux rates were calculated from the 20 chamber measurements obtained during individual sampling events.
Chemical and biological properties of the soil
After the soil respiration measurements were completed for a given sampling period, the soils to the depth from 0-10 cm and 10-25 cm below each PVC collar were collected to determine organic carbon (OC) and total nitrogen (TN), fine root density and microbial lipid concentration (PLFA analysis). Immediately after collection the soil samples were sieved with 2 mm mesh size and all visible fine roots were picked out. Root samples were then washed with distilled water and oven-dried at 65°C for 48 h for biomass determination.
After homogenization of each soil sample, aliquots (c. 2 g) were taken for organic C and total organic N analysis. After air-drying, soil samples were finely grinded with a steel ball mill (Mixer Mill, Retsch MM 200) and dried overnight at 105°C. Soil organic carbon and nitrogen contents were analyzed using a Vario EL III elemental analyzer (Elementar Analysensysteme GmbH, Germany).
Likewise, directly after homogenization of the soil, subsamples of about 10 g were collected separately for phospholipids fatty acid (PLFA) and neutral lipid fatty acid (NLFA) analysis. After removing all visible plant materials, the soil samples were then packed in small glass vials and kept frozen until analyzed. Lipid fatty acid extraction was carried out using frozen soil samples (1.5 g) extracted overnight with a chloroform-methanol citrate buffer mixture (1:2:08) by the modified method described by Bligh and Dyer [41]. The fatty acid 19:0 (nonadecanoic acid methyl ester) was added to the samples as an internal standard. Lipids were separated into neutral, glyco-, and phospholipids using solid phase extraction with silicic acid column (Bond Elut LRC-Si, Varian Agilent Technologies, Santa Clara, CA). Thereafter, the neutral and phospholipids were subjected to a mildalkali methanolysis, and the resulting fatty acid methyl esters were separated by gas chromatography using an Agilent 7890A GC-MS (Varian Agilent Technologies, Santa Clara, CA). The fatty acids were quantified by comparison of the peak areas with those of the standard peak. Standard nomenclature was used to refer to the PLFAs according to the designation described in Zelles [42]. Phospholipids fatty acids i15:0, a15:0, i16:0, 18:1ω7c and cy19:0 were used to represent bacterial biomass [42], while the PLFAs 18:2ω6 and 18:1ω9 were considered to have fungal origin [43,44], and the NLFA 16:1ω5 was used as marker for arbuscular mycorrhizal fungi [45]. The sum of the mentioned PLFAs in addition to PLFAs being not assigned as biomarkers (16:1ω9, 16:1ω7c, 16:1ω5, 16:0, 10Me16:0a and 10Me16:0b) were used to represent microbial biomass [46,47] and expressed as nmol PLFA g-1 dry soil. At our study site, C. lusitanica is infected by arbuscular mycorrhiza.
Statistical analysis
Individual collars were used as the statistical units for analyzing the spatial variation. Statistical differences in the temporal variability of the soil temperature and soil moisture between thinned and control stands were analyzed using student t-test, at 95% confidence interval. A t-test or, if normality was not fulfilled after transformation, a Mann- Whitney Rank Sum test were used to analyze the difference between thinned and control stand in concentrations of fine roots (i.e., fine root biomass), OC and TN, total PLFAs and 16:1ω5 NLFA. For each collar from thinned and control stands the relationships between soil CO2 efflux rate and soil chemical and biological parameters (fine root biomass, OC and TN, PLFAs) was examined with linear regression. The statistical analysis (α 0.05 level) was performed using R 2-11.0 [48] or SigmaPlot version 11 (Systat Software Inc., San Jose, CA, USA).
The effects of thinning, season, soil moisture and soil temperature (and their interaction) on soil CO2 efflux variability were tested by linear mixed effect (LME) model. The model was set up with respiration rate at a collar as dependent variables with treatment (thinned and control) and season (wet and dry) as fixed effects. We nested the random effects of collar numbers within the experimental plot. Because of repeated measurements on the same sampling unit, correlation between the residuals within the same collar was expected. To account for this possible autocorrelation, a first-order continuous autoregressive structure (CAR(1)) error process was incorporated. Plotting the residuals of soil CO2 efflux as a function of soil moisture showed a clear curvature and hence the quadratic effect of soil moisture is also included in the LME model. We used maximum likelihood to calculate parameter estimates and associated standard errors for each fixed effect, with the package nlme using program R Version 2.10.1 [48-50]. We examined residual plots to ensure that variance was homoscedastic and after fitting we have found out that the residual variation of soil CO2 efflux increased with increased fitted values. Thus we used a log transformation of the soil CO2 efflux data to improve homogeneity of variance [50]. We also used nonlinear regression analysis with the objective to describe the dependence of soil respiration on soil moisture or with soil temperature. More details about further statistical approaches can be found in Yohannes et al. [51].
Seasonal variability in soil CO2 efflux under thinned and control stands
For the period of May 2009 to May 2011, Figure1 shows the temporal patterns of air temperature and soil temperature at 0.10 m depth (Figure 1a), precipitation and soil moisture at 0.06 m depth (Figure 1b), as well as the soil CO2 efflux rate (Figure 1c) for the thinned and control stands. The seasonal pattern of the soil temperature followed that of the air temperature (Figure 1a), however, with smaller fluctuations. Likewise, soil moisture exhibited pronounced seasonal variations over the 2 years of observations, mirroring the precipitation pattern (Figure 1b). The highest soil water content of up to 49% were observed towards the end of the long rain period in August 2010, whereas the soil water contents<10% were registered at the end of the dry period in March - April 2011.
Average soil temperatures during the measurement period were 15.9 ± 1.7°C and 15.2 ± 1.5°C at the thinned and control stand, respectively. In general, soil temperature within the thinned stand were significantly higher as compared with the control stand (p=0.005), with higher between stands differences than within the stands (Figure 2). In contrast, there were no significant differences in volumetric soil water content between thinned and control stands (p>0.05). Considering soil moisture, the matrix of within-stand coefficient of variation versus the between-stand coefficient of variation lined up almost equally along the 1:1 line (Figure 2), indicating that the thinning treatment unlike soil temperature had no effect on change in soil moisture regime.
Across the measurement campaign, mean soil CO2 efflux rate varied from 1.5 to 6.9 μmol m-2 s-1 and from 1.3 to 5.2 μmol m-2 s-1 at the thinned and control stand, respectively (Figure 1c) with seasonal minimum in January 2010 and April 2011 and maximum in June 2009 and June 2010. Soil respiration rates over the period of observation were significantly (p<0.001) higher in the thinned than the control stand (Figure 1c), and differences in the flux density appeared to be more pronounced during wet periods. The coefficient of variation in the soil CO2 efflux rate was larger between stands than within stand (Figure 2), indicating the potential influences of stands specific biotic and/or abiotic factors on soil CO2 efflux variability. The coefficient of variation in the seasonal soil CO2 efflux rate ranged from 9% to 40% (thinned stand) and 12% to 48% (control stand). In general, the seasonality of soil CO2 efflux pattern reflects the changes in soil moisture and different significantly between wet and dry periods (Table 3).
| Factor | Coefficient | Standard error | df | F | p |
| Intercept | -1.0582 | 0.1008 | 1 | 2376.6198 | <0.0001 |
| Thinning | 0.1259 | 0.0456 | 1 | 13.8562 | 0.0098 |
| ST | 0.0651 | 0.0057 | 1 | 1162.9606 | <0.0001 |
| SM | 0.0896 | 0.0056 | 1 | 2651.6588 | <0.0001 |
| Season | 0.0662 | 0.0135 | 1 | 920.3257 | <0.0001 |
| SM^2 | -0.0015 | 0.0001 | 1 | 2125.6025 | <0.0001 |
| ST:SM | 0.0004 | 0.0003 | 1 | 1.6307 | 0.2017 |
df, degree of freedom; SM, soil moisture; ST, soil temperature
Table 3: Summary statistics of linear mixed effect model describing the relationship of soil respiration to thinning treatment, covariates soil temperature and volumetric soil moisture content, and interaction term.
A non linear regression shows a significant (p<0.05) effect of soil moisture on soil CO2 efflux rates that explained about 50% and 48% of the variability under thinned and control stand respectively (Figure 3). Soil CO2 efflux rate as a function of soil moisture was fitted by Gaussian curve, which suggested the existence of thresholds in dependency of soil CO2 efflux rate on soil moisture (Figure 3). Soil respiration rates coincidently increased with increasing volumetric soil water content up to 31%, but the opposite was the truth after soil water content exceeded the abovementioned threshold.
Compared with soil moisture, soil temperature had a minor but significant impact on seasonal variability of soil CO2 efflux. Soil temperature explained 17% and 10% of the temporal variability of the soil CO2 efflux rate in thinned and control stands, respectively (Figure 4).
Soil CO2 efflux as related to biological parameters and stand management
The variance components in the LME model analysis indicate that the nested effect of collars around individual tree attributed to 19% of the total variance of the random effect. This shows the likely effect of inherent characteristics (biotic and abiotic) of the soil underneath the collar to the overall soil CO2 efflux variability. Organic carbon concentration in soil collected from each collars in 0-10 and 10-25 cm soil layers differed between the thinned and control stands, with mean values of 114 ± 27 and 40 ± 13 g C kg-1 soil for the former stand and 93 ± 20 and 21 ± 6 g C kg-1 soil for the latter (n=20, p<0.05 in both soil layer). Similarly the mean TN contents from 0-10 and 10-25 cm soil depth were 10 ± 2 and 4 ± 2 g N kg-1 soil for the thinned and 9 ± 2 and 2 ± 0.5 g N kg-1 soil for the control stand, being significantly different (p<0.05) at the 10-25 cm soil depth.
The concentrations of microbial biomarker (nmol total PLFA g-1 dry soil) used as a proxy for microbial biomass assessment were 22% larger (p<0.05) in the soil from 0-10 cm depth sampled from the thinned stand than in those under the control (Figure 5). The soils from the thinned stand also were characterized by larger concentrations of PLFAs being attributed to bacteria (nmol bacterial PLFA g-1 dry soil) and fungi (nmol fungal PLFA g-1 dry soil), being 19% (p<0.05) and 39% (p=0.025), respectively, more than in the soils from the control stand. At both soil depths, soils under the thinned stand contained also larger a concentration of the NLFA16:1ω5 arbuscular mycorrhizal fungi biomarker (p<0.05) (Figure 5).
Figure 5: Total microbial (a), bacterial (b) and fungal markers (c) PLFA concentrations (nmol PLFA g-1 dry soil) and total AMF marker (d) concentration (nmol NLFA g-1 dry soil) collected from thinned and control stand from 0-10 cm (top graph) and 10-25 cm soil depth (bottom graph), n=20. Box plots indicate the distribution by percentiles. The lower and higher part of the box indicates the 25th and 75th percentiles, respectively. The value of the error bars are the 10th and 90th percentile, and the 0th percentile (median) is given by horizontal line within the box. Please note the difference on the scale of Y-axis.
The mean (n=20) fine root biomass was 145 ± 25 (0-10 cm soil depth) and 105 ± 10 (10-25 cm soil depth) at the control stand, being11% (p=0.023) and 12% (p<0.001) respectively smaller as for the thinned stand (not shown).
There was no significant relationship between soil CO2 efflux with OC, fine root biomass, and PLFAs / NLFA analyzed in the soil samples taken directly after the measurement underneath each collars. However, the microbial fatty acids content in 0-10 cm soil depth were positively related with fine root biomass (Pearson’s r=0.74 at p<0.05 and r=0.69 at p<0.05), OC (r=0.52 at p<0.05 and r=0.18 at p<0.05), and with TN (r=0.59 at p<0.05 and r=0.10 at p<0.05); values in parenthesis are for thinned and control stands, respectively.
Seasonal variability of soil CO2 efflux in relation to soil moisture and soil temperature
Not surprisingly, the soil respiration in both stands showed a similar seasonal pattern, with the higher rates observed in wet periods and the lower rates during dry periods. This result is consistent with our previous study conducted in adjacent natural forest [51]. Our finding is also in agreement with results reported for other tropical forests with seasonality in precipitations [25-28]. However, a positive effect of soil moisture on soil respiration could only be identified when it was below 31% volumetric soil moisture content. Soil water contents above this threshold led to a decrease in soil respiration (Figure 4). It should be also noticed that, the threshold for the evergreen Cupressus lusitanica stand reported here, is equal to the threshold of evergreen tree species (Podocarpus falcatus and Prunus africana) examined in the adjacent natural forest and exceeded that for deciduous Croton macrostachys [52]. The decline in soil CO2 efflux rate at extremely wet conditions could possibly result from physiological stress of heterotrophic microorganisms due to oxygen deficiency that decreases decomposition rates of soil organic matter [53]. Alternatively, periods with the most precipitation are associated with conditions of low photosynthetic active radiation. This may decrease carbon assimilation rates and, consequently, the rates of associated autotrophic soil respiration [54].
Although the effect of soil temperature on the seasonal pattern of soil respiration has been widely confirmed by many researchers [10], our result indicated that soil respiration was only slightly positively related to soil temperature. This suggests that soil temperature was not the key determinant of soil respiration at the study site. The apparent weak contribution of soil temperature unlike to soil moisture is partly due to the relatively small temporal temperature fluctuations in this ecosystem, being not sufficient enough to drive seasonal variations in soil respiration [28,51]. Further, at times of high soil temperature soil CO2 efflux was restrained by low soil moisture (Figure 4).
Soil CO2 efflux in relation to CN and biotic factors
Regression analysis of the soil CO2 efflux rates with both fine root biomass, soil OC or TN, and the different microbial parameter sampled underneath the respective collars showed no correlation. The lack of any relationship is surprising as soil CO2 efflux from forest soil derived both from respiration of plant roots and soil microorganisms, positive relationship between soil respiration rate and fine root biomass and/or microbial parameters have been frequently reported [55,56]. In young stands of trembling aspen and paper birch, King et al. [57] found that increased fine root biomass was accompanied by an increase of soil CO2 efflux by 39%. Fine roots influence soil physical and chemical environment via exudation of carbon-rich substances [58]. This argument highlights the decisive role of fine roots to change soil OC concentration which is one of the main determinants of soil CO2 efflux variability [37]. In our case, we speculate that the lack of any relationship can be due to the overriding effect of abiotic parameters in the control of the soil CO2 efflux rate at this particular date. At that time the averaged volumetric soil moisture underneath the individual collars was 33%, and with that above the threshold level of c. 31% as identified in Figure 3. The negative impact of the high soil moisture on soil respiration rates may have leveled off effects of different concentration of the organic substrate or the microbial biomass [59].
Thinning effects on soil CO2 efflux
An averaged soil CO2 efflux rate at the thinned stand was 13% larger than at the control stand. Thinning alters the abiotic stand parameters such as increasing the soil temperature or improving the light conditions due to loss of competitors. Soil temperatures increased with thinning intensity [60-62], and are probably caused by higher insolation to the soil surface under a more open canopy. As soil temperature affects the soil CO2 efflux rates at the investigated stands to a certain extent (Figure 4), the increase in soil temperature might have contributed to the larger soil respiration in the thinned stand. In contrast, soil moisture, the major abiotic driving factor at the stands under study, did not get al. tered by the management impact.
A major reason of any thinning operation is the improvement of the light, nutrient, and water supply to increase stand productivity and obtaining better timber from potential crop trees [63]. After three years of thinning treatment, the basal area increment (at diameter breast height, 1.3 m from the ground) of the experimental trees in the thinned stand was on average 20% larger than trees under study in the control stand. As the soils are highly fertile and water supply is also not limited (in addition to the precipitation, there is lateral water input to the stand due to its downslope position), the major reason for this increased growth rates was rather the improved conditions for incoming photosynthetic active radiation. Larger biomass production requires more nutrient acquisition by the trees, thus leading to larger fine root biomass and larger colonization by arbuscular mycorrhiza, as it can be concluded from the larger concentrations of 16:1ω5 NLFA in the thinned stand soils. Hence, both components of the autotrophic continuum may have enhanced soil respiration at the thinned stand due to higher energy demand, thus increasing the autotrophic respiration [61].
However, fine roots, after their death, are also a source of heterotrophic respiration. Larger concentrations of biomarkers indicative for total microbial biomass (all PLFAs), bacteria (i15:0, a15:0, i16:0, 18:1ω7c and cy19:0 PLFAs) and fungi (18:2ω6 and 18:1ω9 PLFAs) suggesting fuelling of heterotrophic activity by higher substrate supply, i.e. by rhizodeposition, at the thinned stand. Such positive relations between fine roots and microbial biomass parameters have been frequently reported [64]. Also Hwang and Son [65] found larger fine root input to soil after thinning and argued that this was enough organic matter input to offset potential losses of soil OC by soil respiration. This might also explain our observation that despite the higher soil CO2 efflux rates, OC and TN concentrations tended to be even larger at the thinned stand.
In general, our results concur with the findings of other studies reporting that thinning treatments led to elevated soil CO2 efflux rates in plantation forests [66-69]. However, there are also reports showing the opposite effect of thinning on the soil CO2 efflux rate [63,70]. Varying results on the effect of thinning treatment on soil CO2 efflux variability could possibly arise from differences in thinning intensity, the timing of the post-thinning monitoring period, tree species and age of the study trees and sampling design [70-72]. In our study collars were placed randomly around trees as it has been shown that measurement of fluxes in a closer distance to the nearest tree give more reliable estimates of greenhouse gas emissions from a given stand than measuring further from the tree stem [39]. Oshashi et al. [67] designed their sampling points in a similar way and also found larger soil respiration rates of the thinned stand as compared to the control.
In the present study, selective thinning was applied in which only competitors’ trees were removed that found next to trees assigned as potential crop trees. Concilio et al. [69] also carried out selective thinning and measured a 43% and 14% higher soil respiration rate in the thinned areas as in the control of a mixed-conifer forest and a hardwood forest, respectively. According to Son et al. [73] soil respiration was higher at a lightly thinned than at heavily thinned stand. A heavy management impact probably reduces the carbon translocation from plant to soil more, and with that root respiration and the supply of readily available OC source for heterotrophic respiration, as potentially the decomposition of the old organic matter is accelerated by more favorable abiotic conditions. Therefore, differences in thinning intensity might be also one of the reasons for the inconsistence reports among similar studies.
Further, the period when the soil respiration measurements have been done after the thinning might have an influence on the result. In a nine-year old Japanese cedar forest Ohashi et al. [67] reported that 3-4 years after thinning soil respiration was higher in a thinned stand than those of intact stand, but there was no difference 5 years after the thinning. In our result, the differences in average soil CO2 efflux rate between thinned and control plot was larger in the first measurement year than the second. The largest soil CO2 efflux during the early periods since thinning can be attributed to an increased heterotrophic respiration resulted from an accompanied change in the microclimate that favors decomposition of dead tree roots and slashed materials from the thinned trees. At our site, it is also likely that decomposition of dead roots from thinned trees influenced total soil respiration, nevertheless decomposition rate in the tropic is very fast [74] and less likely to prolonged more than a year. Therefore change in soil CO2 efflux between thinned and un-thinned stand particularly after two years after thinning treatment could emanates from both autotrophic and heterotrophic respirations.
Thinning of the C. lusitanica stand was carried out in order to increase productivity of the trees and raise the economic value of the timber. In this study we investigated an important part of the C cycle, the soil CO2 efflux, as influenced by thinning and in connection to the weather variables. The soil respiration rate was on average 13% larger in the thinned stand as it was estimated by the LME model. Soil moisture could have been identified as the key abiotic driver. Due to the pronounced seasonality in precipitation, soil moisture overrides the effect of soil temperature on soil CO2 efflux rate that was found to be minor. While soil moisture did not differ between the thinned and control stands, temperature in soils (10 cm depth) of the former was significantly higher due to higher insolation. This higher soil temperature likely contributed to a certain extent to the higher soil CO2 efflux at the thinned stand. However, it is most likely that the increased in soil respiration is the result of the trees’ response on the silvicultural treatment. Higher fine root concentration of the thinned stand may suggest both, a higher autotrophic respiration and a higher heterotrophic respiration, as is also indicated by higher concentrations of biomarkers representing the microbial biomass. Despite the higher C losses as soil CO2 efflux, the OC and TN concentrations in soil rather tended to increase. Hence, fostering tree growth by thinning obviously increased the organic C input to soil, counter balancing the C losses.
This work was supported by Deutsche Forschungsgesellschaft (DFG) within the project package (PAK 188). We thank Deksiso Bulcha, Getu Tadesse, Temesgen Yohannes, and Awol Assefa for their assistance and support in collecting data in the field. We also thank Christian Rumpf, Elke Eichmann-Prusch, Joanna Weiss, Leopold Sauheitl, Pieter Wiese, Roger-Michael Klatt, Silke Bokeloh and Ulrike Pieper for their laboratory assistance in PLFA and soil analysis. Likewise we are grateful to Frank Schaarschmidt for his advice in statistical analysis.