Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2022)Volume 12, Issue 1
Unripe plantain fruit (Musa paradisiaca) has immense potential as an excellent nutritive source for diabetic patients. Evaluation of its chemical and nutritional properties was carried out as a first step in realizing its food value. Alkaloids, saponins, tannins, phlobatannins, anthraquinones, flavonoids, polyphenols, cardiac glycosides, anthranoids, and reducing sugar in the fruit were assessed by analytical methods. The fruit contained 20%, 4%, 10%, 12.25%, and 65.75% of moisture, ash content, crude fat, protein content, and carbohydrate content, respectively. Additionally, carbohydrate and moisture contents in it were much higher than other parameters studied. The result obtained showed that cardiac glycoside, polyphenols, and reducing sugars were present while alkaloids, saponins, tannins, phlobatannins, anthraquinones, flavonoids and anthranoids were absent. The presence of polyphenols and other medicinal components point to the potential health value of the fruit, while its high carbohydrate composition and appreciable crude fat and protein contents reveal its food value. It is expected also that the phytochemicals can be extracted and successfully encapsulated in diabetic drug formulations to alleviate diabetic conditions.
Unripe plantain; Proximate analysis; Phytochemical screening; Flavonoids; Alkaloids; Natural products
Plantain (Musa paradisiaca), is an herbaceous plant (up to 9 m long) with a robust tree-like pseudo-stem, crown of large elongated oval deep-green leaves (up to 365 cm in length and 61 cm in width), and a prominent midrib. Each plant produces a single inflorescence like drooping spike, and large bracts opening in succession. Fruits are oblong and fleshy (about 5-7 cm long) in wild form and longer in cultivated varieties [1]. It is classified into: Kingdom Plantae, Division Magnoliophyta, Class Liliopsida, Order Zingiberales, Family Musaceae, Genus Musa, and Species paradisiaca. Some interesting researches have been conducted on the plantain fruit. For instance, mature green fruit of Musa paradisiaca was found to improve semen parameter of Wistar rats [2]. Another study revealed that Musa paradisiaca peel extract offers resistance against human lungs cancer invasion [3]. Musa paradisiaca fruit is an excellent source of potassium which helps in maintaining the proper function of muscles and preventing muscle spasm. It also helps to maintain blood pressure of an individual and reduces the risk of stroke [4]. Plantain diet has a mean arterial blood pressure lowering as well as onset prevention effects in rats with elevated blood pressure induced by Desoxycorticosterone Acetate (DOCA) administration [5].
Plantain has a good antioxidant activity in human diet. Methanol extract obtained from Musa paradisiaca protects against colorectal cancer risk [6], while the extracts from its flowers possess antioxidant properties and thereby stabilizes the free radicals formed during various metabolic process of body and hence help in maintaining the stability of DNA and proteins of human diet [7]. Musa paradisiaca phytochemicals and herbs have also been reported to be an effective laxative [8] and for ulcer treatment [9]. Musa paradisiaca stalk when used as dietary supplement in the treatment of diabetics, the reduction of hyperglycermia was observed [10]. Leaf juice of Musa paradisiaca has also been used in treatment of wounds, cuts and insect bites [11]. Sap of the plant has been used as a remedy for epilepsy, hysteria, dysentery and diarrhea, with its root as an anthelminthic [8]. Musa paradisiaca leaf is used to wrap steamed bean pudding made from a mixture of beans with onions, fish and boiled egg, the pseudo-stems yield a pulp that can be made into brown paper products, while fertilizers are made from dried chopped plantain stem and leaves. The shoot is considered rich in fiber hence a good remedy for avoiding constipation. The stem and peel juice are used as a first aid for burns and minor abrasions and help to balance haemostasis. Methanolic root extract of Musa paradisiaca lowers cholesterol in diabetic rat [12], while the green fruit of Musa paradisiaca had been reported to have hypoglycemic effect [13].
Musa paradisiaca has various pharmacological properties such as its methanolic root extract which has hypo-cholesterol-anemic activity [3] and serves as antidiabetic agent [14]. The study by Adeyemi and Oladiji [15] on the compositional changes in banana fruits during ripening revealed that moisture and ash content values were 73.47% and 0.68%; 77.19% and 0.80%; 79.22% and 0.78% in unripe, ripe, and overripe banana fruits, respectively. They also concluded that ash and moisture content in plantain increases with ripening.
Akpabio et al. [16] examined the phytochemicals present in Musa paradisiaca and banana (Musa sapientum) pseudo-stems oil and stated that the phytochemical composition in mg/100 g of plantain and banana pseudo-stems oil constitutes oxalates (66.28 ± 2.01, 41.56 ± 2.55); phytates (3.78 ± 0.05, 3.23 ± 0.33); tannins (7.99 ± 0.26, 6.55 ± 0.33); and cyanogenic glycosides (1.47 ± 0.14, 1.44 ± 0.33) respectively. Flavonoids, saponins and alkaloids were present in both varieties, but steroids were absent. The values of lignin ranged between 2.28 and 7.30% pseudo- stems, the carbohydrate between 39.71 and 62.21 mg/100 g for plantain and 42.13 and 93.82 mg/100 g for banana pseudo- stems, while the pentosan content for both ranged between 6.67 and 11.73 mg/100 g. They stated that plantain pseudo- stems contain higher values of the phytochemicals than their banana counterparts except the pentosan content; both contain high amount of carbohydrate which can be exploited for the preparation of pulp for paper making and production of cellulose derivatives and sugar.
A study on the effect of ripening on the phytochemical constituents and antioxidant property of plantain revealed that ripening depleted the total phenolics and flavonoid content of plantain [17]. Unripe plantain extract exhibited a significantly higher free radical scavenging, iron (II) chelating and ferric reducing effect than the ripe extract. Unripe plantain extract was found to offer a marked inhibitory effect against pro-oxidant induced deoxyribose degradation and lipid peroxidation than the ripe extract. Phyto-analysis of methanolic and ethanolic extracts of the pulp of Musa paradisiaca var Bontha extracts revealed that it contains alkaloids, flavonoids, glycosides, saponins, steroids, tannins and xanthoproteins [18]. They recorded that the phytochemicals present in the extract were responsible for the activities displayed by the extract against microorganism; hence Musa paradisiaca can be used as a source of therapeutics.
The green fruit of Musa paradisiaca has also been reported to have hypoglycemic effect due to stimulation of insulin production and glucose utilization [13]. Agarwal et al. [19] reported the wound healing activity of both methanolic and aqueous extracts of plantain (M. sapientum var. paradisiaca) in rats. Both extracts were found to decrease the wound area, scar area and lipid peroxidation. The effects were attributed to the antioxidant property of plantain. Most diabetic patients are also advised to consume unripe plantain for its phytochemical contents as it helps to reduce the blood sugar. Therefore, the aim of carrying out this research on proximate and phytochemical analysis of Musa paradisiaca is to enable researchers to know the dietary values present in Musa paradisiaca and hence encourage extraction of the phytochemicals present in it and encapsulate it in diabetic drugs. A review of relevant literature reveals that studies have been carried out on the phytochemicals of plantain, but no studies has been conducted on the unique Musa paradisiaca species in Cross River State, Nigeria.
In this study, aqueous and petroleum ether extracts of unripe plantain fruit were obtained in Cross River State, Nigeria, and proximate composition was determined. The phytochemical parameters in the aqueous and petroleum ether extract of the unripe plantain and the significance of the proximate and phytochemical composition in nutrition and health were evaluated. It is envisaged that the outcome of this study will provide substantial information on the nutritional contents of the unique plantain variety in in Cross River State, Nigeria, that can add to already existing knowledge on the fruit worldwide and guide its further deployment in various food processing aspects and drug development Figure 1.
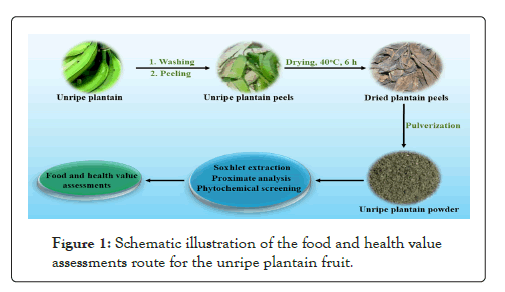
Figure 1: Schematic illustration of the food and health value assessments route for the unripe plantain fruit.
Materials
Sulphuric acid, boric acid, sodium hydroxide, distilled water, ammonia, hydrochloric acid, perchloric acid, acetone and anthrone reagent. Weighing balance, solvent extractor, oven, crucible, thermometer, conical flask, retort stand, manual blender, filter paper, muffle furnace, glass funnel, desiccator, beaker, pipette, test tube, evaporation dish, sample bottle, hot plate, and indicator were used for the proximate analysis. Ferric chloride, magnesium metal, hydrochloric acid, potassium ferrocyanide, Fehling’s solution, potassium hydroxide, acetic acid, toluene and benzene were used for phytochemical screening.
Sample collection and preparation
Fresh fruits of Musa paradisiaca were bought from akpabuyo local government of cross river state, nigeria and its identity confirmed by a plant taxonomist. The unripe plantain fruits were washed with distilled water, processed by peeling the back, chopped into smaller pieces using knife with steel blade and spread separately on a wooden board. It was then placed in oven pre-set at 45°C for 6 h to dry off the moisture content. After oven drying, the samples were ground into powder with the aid of a manual blender. The powder obtained was placed in a rubber container, properly labelled and stored safely in the desiccator for use.
Sample extraction
Solvents used for extraction were distilled water and petroleum ether. For water-based extraction, 20 g of the ground sample was weighed, packed into the extraction thimble and fitted into Soxhlet sets. The Soxhlet apparatus was heated under reflux using heating mantle for 6 h until extractant was colorless. The same process was repeated for the sample using petroleum ether. The extracts obtained were transferred into reagent bottles after cooling and kept safely in the laboratory for use.
Proximate analysis
For the proximate analysis, the content of moisture, crude protein, fibre, ash, fat, and carbohydrate of the sample were carried out using the method described by the Association of Official Analytical Chemists [20].
Determination of moisture content: 5 g sample of the powder was weighed into a beaker and placed in an oven for about 6 h at a temperature of 45°C. The weight was taken after drying. The loss in weight was expressed as a percentage of the initial weight. Thus, the difference in weight indicates the amount of water contained in the sample.
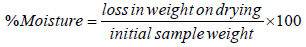
Determination of ash content: The ash content was determined from the loss in weight after ash formation of 5 g of the ground sample at 50°C using a muffle furnace. This temperature was considered high enough to allow organic matter to be burnt off without appreciable decomposition of the ash content.
Determination of crude fiber content: Ground sample weighing 5 g was put into a beaker to which 50 ml of 1.25% H2SO4 solution was added and made up to 200 ml with distilled water and stirred. The mixture was heated with continuous stirring for 30 min and allowed to cool and settled. Distilled water was added and allowed to settle then decanted. Decantation was repeated for six times consecutively to make the mixture acid-free. 50 ml of 1.25% NaOH was added to the mixture and made up to 200 ml with distilled water in a beaker, and heated for 30 min with continuous stirring after which it was cooled and allowed to settle. Distilled water was added and decanted for six times consecutively. The mixture was filtered with filter paper and kept for 45 min for water to drain completely, and the weights taken.
Determination of crude fat content5 g of the ground sample was weighed into a beaker of known weight. The crude fat was extracted in a soxhlet extractor using petroleum ether (B.P 40°C-60°C) as solvent. When the extraction was completed, the solvent was evaporated by placing in the oven at a temperature of 150°C. The weight of extract left was taken as the weight of crude fat in the samples.
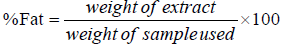
Determination of crude protein
5 g of the sample was weighed into a beaker, 30 ml of concentrated sulphuric acid, 2 g of copper sulphate and 16 g of sodium sulphate salt were added and shaken for few minutes. Thereafter, the beaker was heated with continuous stirring until a clear green solution was obtained. This was dissolved in distilled water and made up to 100 ml in a volumetric flask. 12.5 ml of the digest were measured into a semi-micro Kjeldahl Markham distillation apparatus and treated with 12.5 ml of 1.25% of sodium hydroxide (NaOH) solution. This was distilled with 10 ml of boric acid and double indicator. The distillate was then titrated with 0.1% HCl solution until a light pink end point was obtained. Blank titration was also carried out in similar manner. Distillation was carried out in triplicate and the percentage nitrogen obtained by appropriate calculation below.
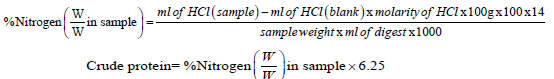
Determination of carbohydrate: The carbohydrate content of the unripe plantain fruit was determined from the difference obtained after subtracting total organic nitrogen, fat, ash and fiber from the total dry matter.
Phytochemical screening
Phytochemical screening procedures carried out were adopted from previous work on plant analysis [21]. This analysis determined the biologically active non-nutritive compound that is responsible for the flavour, colour and characteristics of plants parts. The extracts were used for the following plant constituents: cardiac glycosides, alkaloids, saponins, tannins, flavonoids, polyphenols, reducing compounds, phlobatannins, anthranoids, and anthraquinones using the methods described in the following sub-sections.
Test for cardiac glycoside: The cardiac glycoside content was determined by dissolving 2 ml of aqueous plant extracts in 2 ml of chloroform. Concentrated sulphuric acid was carefully added to form a lower layer. A reddish brown colour at the interface indicated the presence of glycone of the cardiac glycosides.
Test for alkaloids: For the determination of the alkaloid content, 2 ml aqueous plant extracts were put in a test tube and treated with 10 ml of 1% hydrochloric acid for 10 min in a water bath. 1 ml of the filtrate was treated with a drop of Mayer’s reagent. Turbidity or precipitate with either of this reagent was taken as presence of alkaloids [21].
Test for saponins: 2 ml aqueous plant extracts were shaken with distilled water in a test tube, frothing which persist on warming was taken as evidence for the presence of saponins according to a previous study [21].
Test for tannins: For the determination of the tannin content, 2 ml of aqueous extracts was stirred with 10 ml of distilled water. This was filtered and 1% ferric chloride added to the filtrate. Blackish-blue precipitate indicates the presence of hydrolysable tannins (callic) while the blackish-green precipitate indicated the presence of condensed tannins (cothecol).
Test for flavonoids: The flavonoid content was determined by adding 2 ml of aqueous extracts to a few pieces of magnesium metal and concentrated hydrochloric acid was added. The formation of orange, red crimson or magenta was taken as evidence for the presence of flavonoids.
Test for polyphenols: About 2 ml aqueous extracts with 10 ml of distilled water was heated for 30 min. 1 ml of 1% FeCl2 was added to the mixture and followed by the addition of 1 ml of 1% potassium ferrocyanide to the solution. This was filtered and the formation of a green-blue colour indicates the presence of polyphenols.
Test for reducing sugar: 2 ml aqueous plant extracts were placed in a test tube. Then, 5 ml Fehling’s solution was added. The mixture was heated in a water bath. The formation of a brick red precipitate was taken as evidence for the presence of a reducing sugar.
Test for phlobatannins: Phlobatannins was determined by boiling 2 ml aqueous plant extracts with 1% HCl. The deposition of a red precipitate was taken as evidence for the presence of phlobatannins [22].
Test for anthranoids: About 2 ml aqueous plant extracts was boiled with 5 ml of potassium hydroxide (KOH). The solution was filtered through glass wool. The filtrate was treated with 1% acetic acid and the resulted solution was mixed with toluene. The upper layer was transferred to another test tube and potassium added. The presence of a red colour indicated the presence of anthranoids.
Test for anthraquinones: 2 ml of aqueous plant extracts were shaken with 10 ml of benzene. This was filtered and 5 ml of 1% ammonia solution was added to the filtrate. The mixture was shaken and the presence of a pink, red or violet colour in ammoniacal (lower) phase indicated the presence of anthraquinones [23].
Proximate composition
Determination of proximate composition of food is an important index in assessing nutritional potential of crops. Table 1 depicts the nutrient composition present in the plantain cultivar considered in this study
Moisture content: The moisture content in the plantain fruit is quite high with a value of 20% as shown in Table 1. High value for moisture content was also found by Adeyemi et al [15]. The result obtained in this research for moisture content is comparable to 13.28%‒20.38% and 10%‒18.30% reported by Akinsanmi et al. [24] and Oko et al. [25], respectively. Moisture content gives an indication of its freshness and shelf life. High moisture content can subject food items to increased microbial spoilage and short shelf life, which can lead to its deterioration [26]. However previous studies have shown that moisture content of 20% or less is suitable for a stable shelf life of plantain. [25]. Moreso, moisture content in unripe plantain flour makes it a good binder and composite flour in food and baking industries [25].
Ash content: The ash content obtained in this research was 4% as shown in Table 1. This value is higher than 0.68% and 2.68% obtained by Adeyemi et al. [15] and Zakpaa et al. [27], but in close range to 3.23% obtained by Uzama et al. [28] for unripe plantain fruit. Furthermore, it is low compared to the value of 5.50% obtained by Eleazu et al. [29] and 8.93% obtained by Eleazu and Eleazu [30]. However, a similar value of 4.71% was reported by Auta and Kumurya [31]. The low value of the ash content is indicative of low mineral (especially the macro minerals) content of the plantain.
Protein content: The protein content obtained was 12.25% as also shown in Table 1. The value is greater than that of 2.68% and 3.60%, obtained by Zakpaa et al. [27], and Ojure and Quadri [32]. However, it is in close range with the values of 7.89%, 7.24%, and 11.47% reported by Akinsanmi et al. [24], Oko et al. [25], and Adeolu and Enesi [33], respectively. The protein content obtained in this study is also and lower than 14.50% reported by Fingolo et al [34]. Protein content present in unripe plantain is an essential component of diet needed for survival of animals and human being. It functions in nutrition to supply adequate amount of required amino acids. The crude protein value of 12.25% suggests that unripe plantain is a moderate source of protein. Pamela et al. [35] suggested that protein from plant sources have lower quantity, but their combination with many other sources of protein such as animal protein may result in equivalent nutritional value.
Lipid content: Lipid content in unripe Musa paradisiaca fruit was 8.00% as shown in Table 1. This value agrees with 8.52% reported by Eleazu and Eleazu [30]. However, it is greater than 5.23%, 4.80%, 4.04%, and 1.00% reported by Akinsanmi et al. [24], Akubor and Ishiwu [36], Fingolo et al. [34], and Adubofuor et al. [37], respectively. Low fat content will increase the storage life of the flour by reducing the chances of developing rancidity; it is also advantageous in absorption of glucose and fat. Many body functions depend on lipids. Lipids provides excellent source of energy and enhance transport of fat-soluble vitamins, insulate and protect internal tissues and contribute to vital cell processes [35].
Fibre content: Fibre content of 10% as shown in Table 1 obtained was slightly higher than the value of 8.47% and 6.20% obtained by Adeolu and Enesi [33], and Eleazu et al. [38], respectively. However, values of 3.09%, 1.62%, and 1.87% were obtained by Akubor and Ishiwu [36], Auta and Kumurya [31], and Uzama et al. [28], respectively, for the fibre content of the plantain species they studied. Dietary fibre is increasingly recognized as a useful tool for the control of oxidative processes in food products and as functional food ingredient. In addition, dietary fibre decreases the absorption of cholesterol from the gut in addition to delaying the digestion and conversion of starch to simple sugars, an important factor in the management of diabetes. Although crude fibre enhances digestibility, its presence in high levels can cause intestinal irritation, lower digestibility and decrease nutrient utilization [39]. Dietary fibre also functions in the protection against cardiovascular disease, colorectal cancer, and obesity. Thus, the high fibre content of the unripe plantain flour as observed in this study infers that the unripe plantain could be effectively utilized in colorectal cancers and weight reduction in obese individuals [38]. High fiber content in diets have been reported to result in increased removal of potential mutagens, steroids and xenobotics by binding or absorbing to dietary fibre components and thereby aids digestion; hence unripe plantain has health-promoting benefits for livestock and fish farming.
Carbohydrate content: Carbohydrate content in the sample was obtained to be 65.75% (Table 1). This value was low compared to 91.16% and 88.20% by Zakpaa et al. [27], but comparable to the value of 67% and 69.96% recorded by Ojure, M.A and Quadri [32] and Oko et al. [25], respectively. High carbohydrate content in the unripe plantain indicates that it can serve as a good source of energy for man and animals [8]. The value of carbohydrate obtained in the unripe plantain flour is due to the presence of large amount of starch and low sugar in its green stage [29]. This carbohydrate content is one of the contributing factors for the efficacy of Musa paradisiaca as an anti-diabetic agent [39].
| Parameter | Percentage composition (%) |
|---|---|
| Ash Content | 4.00 |
| Moisture Content | 20.00Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â |
| Fibre Content | 10.00 |
| Lipid Content | 8.00 |
| Crude Protein | 12.25 |
| Total Carbohydrate | 65.75 |
Table 1: Proximate composition of unripe fruit of Musa paradisiaca from Cross River State, Nigeria.
Phytochemical screening
The results of the phytochemical screening of Musa paradisiaca are shown in Table 2. Many compounds that occur in plant tissues are quite labile and almost inevitably may undergo change during extraction. Polarity of the solvent used for extraction may influence the group of bioactive compounds obtained from the plant material [40]. The different phytochemicals present in the unripe fruit extract of the plant like phenols, cardiac glycoside, reducing sugars etc. gives the possibility of expected pharmacological activities [7]. Antibacterial activity of Musa paradisiaca can be exploited for the treatment of infections caused by the test organism at relatively low doses [41].
Alkaloids, saponins, and flavonoids: Results of alkaloids, saponins, and flavonoids for petroleum ether and water extract in this study are presented in Table 2. The results showed negative for alkaloids in both water and petroleum ether extract, hence the absence of alkaloids. Alkaloids are derived from plant sources. They are basic and contain one or more of nitrogen atoms usually in a heterocyclic ring. Some are poisonous and some are used as drugs [22]. The result shows negative for saponins. Saponins are naturally occurring highly complex glycosides which occur in plants. They are used as foaming agents, used for detergents, used in fire extinguishers as a foam producer and also in industry and mining for things such as ore separation. Flavonoids was absent in the petroleum ether and water extract. Flavonoids are mainly water-soluble compounds, which are phenolics and change in colour when treated with base or ammonia.
| Parameter | Water Extract | Petroleum Ether Extract |
|---|---|---|
| Alkaloids | - | - |
| Saponins | - | - |
| Flavonoids | - | - |
| Polyphenols | ++ | ++ |
| Phlobatannins | - | - |
| Anthranoids | - | - |
| Anthraquinones | - | - |
| Cardiac glycosides | - | + |
| Tannins | - | - |
| Reducing sugar | ++ | ++ |
Table 2: Qualitative phytochemical screening of unripe fruit of Musa paradisiaca from Cross River State, Nigeria.
Polyphenols: The result obtained for the petroleum ether and water extract indicated the presence of polyphenols (Table 2). Polyphenols are compounds that have hydroxyl group directly attached to benzene ring. It helps in contracting certain hemorrhages. Polyphenols are product of the secondary metabolism that originates from two main synthetic pathways: shikimic acid and acetate-malonate pathways. These pathways are derived from glucose metabolism in plants. Polyphenols inhibit the activity of digestive enzymes such as trypsin and amylase. The inhibition of these enzymes stimulates hypoglycemic action in some medicinal plants [38]. Large number of polyphenols present in unripe plantain implies that it is useful in the treatment of diseases involving free radical reactions [29].
Phlobatannins, anthranoids and anthraquinones: The results obtained for phlobatannins in petroleum ether and water extract were negative (Table 2), since there was no colour change or deposition of precipitate. Phlobatannins were absent. The result reveals the absence of anthranoids. Naturally occurring anthranoids are oxo, hydroxyl and hydroxyl-oxo derivatives of anthracene. Anthranoids containing plants are used as laxatives. The results for the petroleum ether and water extract showed the absence of anthraquinones. Anthraquinones is a yellow insoluble powder derived from anthracine and often used as a dye.
Cardiac glycoside, tannins and reducing sugar: The result of phytochemical screening revealed the presence of cardiac glycoside in the petroleum ether extract and its absence in the water extract (Table 2). Cardiac glycosides are naturally occurring glycosides. It is used to strengthen weakened heart in order for the heart to work efficiently. Glycoside detected are used in the treatment of congestive heart failure and cardiac arrhythmias, it also protects the heart from coronary heart disease [39]. The results in Table 2 also show negative for tannins indicating the absence of tannins. Tannins are essential product for plant defenses and they add to the nutritive value of many foods consumed by man. Moreover, the results revealed the presence of high amount of reducing sugar in the petroleum and water extract (Table 2). Insoluble sugars function as structural material in the cell walls of plants and bacteria and in the connective tissue. Sugar polymers help to lubricate skeletal joints. reducing sugar which is useful in the management of diabetes, a disease in which blood glucose levels are dangerously elevated by a failure to produce enough insulin (Type 1 diabetes) or by an inability to respond to insulin (Type 2 diabetes).
The results generally reveal the presence of reducing sugar in both aqueous and petroleum ether extract but cardiac glycoside is found only in the petroleum ether extract (Table 2). This result is similar to the findings of [40] in which cardiac glycoside was identified in the petroleum ether extract and Polyphenols was present in aqueous extract of Musa acuminata bract. The level of detection of these components depended on the method of preparation of the sample, solvent used, and the temperature [39]. The results also show that the petroleum ether extract has a higher level of detection as it is an organic solvent; this is shown in the presence of cardiac of glycoside. The non- detection of most of the components in the extract as compared to other researchers was due to the organic solvent (petroleum ether extract) used in the extraction, as compared to previous researchers who made use of ethanol [40], methanol [41], and ethyl acetate and chloroform extract [29]. The phytochemical screening method employed in this study was qualitative.
The amount of increased access to modern energy services gained through modern bioenergy was measured in terms of energy and household numbers. The number of households using bioenergy (both modern bioenergy and traditional biomass) was measured in this case [18,22]. Improved technologies considered were based on the definition of modern energy services for cooking (energy efficiency and safety to human health) (18) and based on the efficient and safe combustions [25]. Improved cookstoves considered were both closed stoves with chimneys and also open stoves or fires with chimneys or hoods [26]. But open stoves or fires with no chimney or hood were excluded. Improved cookstoves considered were those which have energy efficiency higher than 20-30 per cent and their flue gases are released distantly from their users.
Both biogas feedstock productivities and processing efficiencies by feedstock were calculated [27,28]. The amount of biogas end product by mass/volume or energy content was also calculated [29]. Furthermore, the production cost per unit of bioenergy was measured.
Herfindahl Index (HI) was calculated to observe change in diversity of TPES in the country due to bioenergy. Data on TPES of Ethiopia was based on the energy balance of the international energy agency [30]. The Herfindahl Index (HI) was calculated using the following formula. For this calculation, different sources of energy generations in the country were obtained.
Where: Si=Share of energy sources in TPES and n=Number of energy sources in TPES. The HI can range from 0 to 1. HI=0 when n=∞, HI=1 when n=1. Therefore, a smaller index, closer to 0, indicates higher energy diversity.
Phytochemical screening of unripe plantain fruit (Musa paradisiaca) has been extensively analyzed. The results of the proximate analysis show the presence of carbohydrate and moisture content in large quantity while protein, ash, crude fibre, and crude fat were present in low quantities. The results of the analysis of the phytochemical screening show the presence of cardiac glycoside, polyphenols and reducing sugars in petroleum ether extract and absence of alkaloids, saponins, flavonoids, phlobatannins, anthranoids, anthraquinones, and tannins. The results obtained further reveal that polyphenols and reducing sugars are present in large quantities while alkaloids, saponins, flavonoids, phlobatannins, anthranoids, anthraquinones, cardiac glycosides, and tannins are absent in the water extract. Polyphenols are needed to combat various kinds of disease in humans; thus, efforts should be directed towards harnessing their potentials in drug formulation and development.
The authors appreciate the entire staff of the Department of Pure and Applied Chemistry, University of Calabar, Nigeria for providing free access to equipment and facilities used in this study.
The authors declare no conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Peter BD, Umo FE, Akakuru OU (2022) Food and Health Value Potentials of Unripe Plantain (Musa paradisiaca )from its Chemical and Nutritional Compositions. J Phys Chem Biophys.12:320.
Received: 04-Jan-2022, Manuscript No. JPCB-22-15353 ; Editor assigned: 07-Jan-2022, Pre QC No. JPCB-22-15353 (PQ); Reviewed: 21-Jan-2022, QC No. JPCB-22-15353 ; Revised: 26-Jan-2022, Manuscript No. JPCB-22-15353 (R); Published: 02-Feb-2022 , DOI: 10.35248/2161-0398-22.12.320
Copyright: �© 2022 Peter BD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.