Journal of Women's Health Care
Open Access
ISSN: 2167-0420
ISSN: 2167-0420
Research Article - (2022)Volume 11, Issue 5
Objective: Fitness is a marker of physiological and mental health. Purposes of this pilot study were to assess the feasibility of collecting health and fitness data from women with polycystic ovary syndrome (PCOS) and explore possible associations between anthropometrics, PCOS biomarkers, health-related quality-of-life (HRQoL), and depressive symptoms with that of fitness and self-reported physical activity levels among women with PCOS. Methods: A sample of women with PCOS (n=15) were recruited via flyers and the snowball method. Participants completed surveys, anthropometrics, a dual energy x-ray absorptiometry scan, blood work, and a fitness assessment. Data were statistically analyzed using Spearman correlations. Results: Feasibility measures of recruitment and retention rates were 83% and 100%, respectively. Participants [age 25.9 (± 6.2), mostly White (80%), single (60%), and employed full-time (67%)] were categorized as obese (BMI 32.2kg/m2 ± 8.3, percent body fat 41.1% ± 8.1) with ≤ 1 comorbidity. Most participants were not regularly physically active and had high free testosterone levels (7.6pg/mL ± 4.3), elevated high-density lipoprotein (63.2mg/dL ± 12.9), fair cardiovascular capacity (36.8 ± 10.6), and below average muscular strength/endurance (15.4PU ± 4.0; 21.3CU ± 18.2). The following statistically significant and strong associations were found: 1) VO2 max with percent body fat (-0.59; p=0.02), sex hormone binding globulin (0.73; p=0.00), HRQoL (0.72; p=0.00), and depressive symptoms (-0.67; p=0.00), 2) abdominal strength with BMI (-0.66; p=0.01) and HDL (0.59; p=0.02), 3) physical activity level with percent body fat (0.72; p=0.00), and 4) resistance training with LDL (0.52; p=0.05). Conclusion: Collecting health and fitness data from women with PCOS is a feasible research approach. Randomized controlled trials in which health and fitness data are collected from women with PCOS are needed to confirm possible associations between fitness and PCOS clinical features.
Polycystic ovary syndrome, Anthropometrics, Biomarkers, Central adiposity, Fitness, Fitness testing, Physical activity, Exercise
Polycystic ovary syndrome (PCOS), the most common chronic endocrinopathy among women, has a prevalence that ranges between 15-21% [1]. Common signs/symptoms include subfertility, insulin resistance, dyslipidemia, hirsutism, and lethargy, placing women with PCOS at risk for comorbid conditions such as diabetes, cardiovascular disease, reproductive cancers, and depression and resulting in reduced health-related quality-of-life (HRQoL) [2]. Unfortunately, a PCOS diagnosis is often missed or delayed, as its presentation can widely vary, thus affecting treatment recommendations [3].
According to Rotterdam criteria, PCOS is diagnosed when two of three characteristics exist: clinical or biochemical hyperandrogenism, ovulatory dysfunction, and/or ultrasound evidence of polycystic ovaries [4]. Using all possible combinations, this heterogenous condition produces four phenotypes. For example, some women with PCOS may be obese; others lean and some women with PCOS may have hirsutism, while others may not. Such differences in physical presentation confound PCOS diagnosis and treatment. However, the risk for comorbid conditions holds constant regardless of phenotype due to factors such as central adiposity (affecting 50% of women) and dyslipidemia (affecting 70% of women), which are both independent of total bodyfat percentage [5].
Lifestyle changes, such as exercise, are recommended as first-line treatment by the Endocrine Society Clinical Practice Guidelines for PCOS. However, research indicates that less than 60% of women with PCOS engage in regular physical activity and more than 25% are sedentary [6]. Fitness (the sum attributes of cardiorespiratory capacity, muscular strength and endurance, and flexibility), is a marker of physical and mental health and people who are fit have lower mortality from any cause as compared to those who are not fit [7]. To date, there are no research studies about fitness among women with PCOS as an indicator of PCOS clinical features and health risk. As such, we conducted a pilot study to assess the feasibility of collecting health and fitness information among women with PCOS and to explore possible associations between anthropometrics, PCOS biomarkers, HRQoL, and depressive symptoms among women with PCOS with that of fitness and selfreported physical activity levels.
Theoretical Model
A theoretical PCOS physiological model guided this study (Figure 1). PCOS is multifactorial, as genetic alterations, epigenetic modifications, and environmental factors contribute to development or worsening of the clinical manifestations [8]. Genetic origin of PCOS is confirmed by a hereditary factor observed in first-degree relatives, as women who have mothers or sisters with PCOS are at increased risk (35–40%) of developing the condition. In addition to a genetic basis, epigenetic alterations (e.g., DNA methylation, noncoding RNA regulation) play a central role in PCOS outcomes, including phenotypic difference, by dynamically and reversibly controlling gene expression during childhood and into adulthood. Environmental factors (e.g., nutrition, physical activity, smoking) combined with genetic predisposition can change the epigenome landscape leading to (epi)genetic susceptibility for developing PCOS throughout life [9].
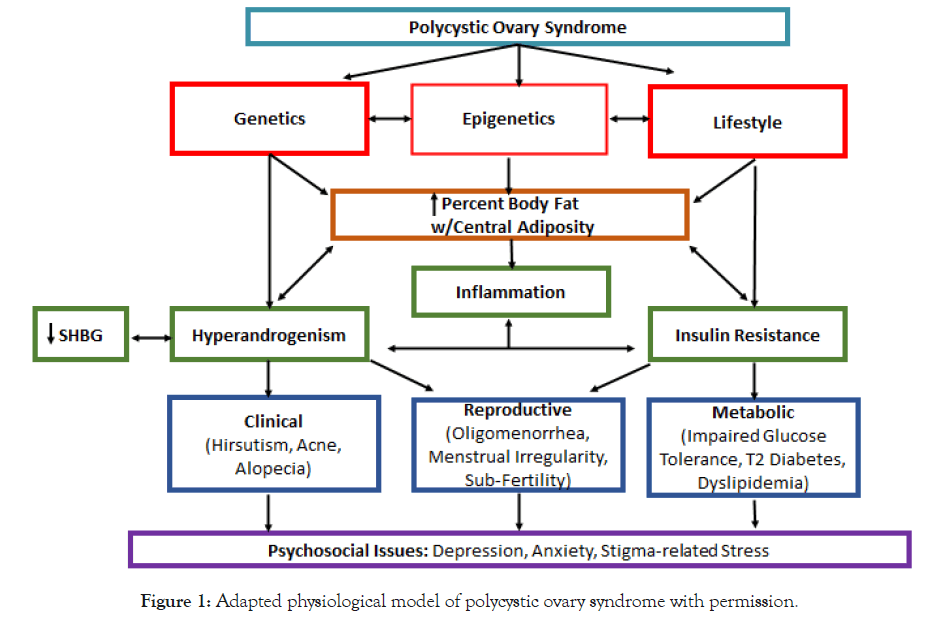
Figure 1. Adapted physiological model of polycystic ovary syndrome with permission.
Obesity is a common characteristic of women with PCOS, and affects clinical manifestations such as hyperandrogenism, insulin resistance, and dyslipidemia. Visceral fat, bodyfat located within the abdominal cavity, is a storage site for circulating androgen. Androgen is also present in plasma as free or unbound testosterone. Further, hyperinsulinemia due to insulin resistance also contributes to excess androgen, as hyperinsulinemia decreases the total amount of sex hormone binding globulin (SHBG), a glycoprotein that binds with androgen. Androgen is also produced in excess by the adrenal glands in most women with PCOS [10] (Figure 1).
Study Overview
Pilot study participants (n=15) were recruited using flyers and the snowball method. Inclusion criteria were women aged 18-42 with a medically confirmed PCOS diagnosis. The number of recruited participants for this pilot study met the investigators’ a-priori target goal. Exclusion criteria included medically induced menopause, cardiovascular disease, orthopedic injury, and pregnancy. Exclusion criteria were identified during telephone or email prescreening using the Physical Activity Readiness Questionnaire. When participants met requirements and provided medical confirmation of a PCOS diagnosis via secured email, they were scheduled an appointment at a university-based clinical exercise research center. Upon arrival at the research center, the study purpose, benefits, and risks were explained to potential participants, and their questions were answered. After informed consent, the following measures were obtained: anthropometrics, venipuncture for laboratory panel, questionnaires, dual energy x-ray absorptiometry (DEXA), estimated oxygen consumption, muscular fitness, and flexibility. Participants received a $50 gift card as an incentive. Research protocols were approved by the university’s Institutional Review Board [11].
Feasibility Measures
Feasibility measures included recruitment and retention rates and participant feedback. The recruitment rate was calculated by dividing the total number of participants who entered the study by the number of participants who answered the call for participants, and then multiplying by 100%. The retention rate was calculated by dividing by the number of participants completing all phases of the study by the number of participants entering the study, and then multiplying by 100%. At the end of each fitness assessment, participants were asked three open-ended questions (see supplementary material) to assess participant feedback about the research process.
Anthropometrics
Anthropometric measurements included height (measured to nearest 0.1cm using a wall-mounted stadiometer), weight (measured twice and to nearest 0.1 kg using an electronic scale), and waist and hip circumferences (measured twice to nearest 0.1 cm using a measuring tape). Body mass index was calculated with the formula [BMI = weight (kg)/height(m)2)]. Waist-to-hip ratio was determined by dividing waist circumference by hip girth. Percent body fat and lean muscle mass were determined using the Lunar fan-beam dual energy X-ray absorptiometry (DEXA) scanner (GE Healthcare model 8743, Waukesha, WI; encore version 15 software used for analyses). DEXA is spectral imaging involving less than two days' exposure to natural background radiation, with demonstrated reliability of an intraclass correlation of R = 0.99 [12].
Laboratory-derived Data
After fasting for at least eight hours before the appointment, participants received venipuncture for a laboratory panel including free testosterone, SHBG, and lipid profile. Trained personnel followed standard protocol for venipuncture to collect approximately 10mL of blood. The specimens were processed at the Lexington Medical Center (West Columbia, SC). Results were confidentially sent to the principal investigator at the UofSC College of Nursing. Free testosterone is used to diagnose androgen excess not due to other rare disorders. SHBG is a PCOS and insulin resistance biomarker. A lipid profile was collected to assess for dyslipidemia.
Questionnaire-derived Data
Demographics: The demographic questionnaire included age, race, educational attainment, marital status, number of children, employment, insurance, geographic residence, and comorbid conditions.
Health-Related Quality-of-Life: PCOS-specific HRQoL was measured using the Polycystic Ovary Syndrome Questionnaire (PCOSQ-50). The PCOSQ-50 has been reported to have a construct validity of 0.92 and test-retest reliability of 0.91 [13]. It includes 50 items in six domains: psychosocial/emotional, fertility, sexual function, obesity/menstrual disorders, hirsutism, and coping. Answers are given on a 5-point Likert scale ranging from 1 = always (worst condition) to 5 = never (best condition). A score for each domain was calculated as the sum of all answered items in the domain divided by the number of answered items. The total PCOSQ-50 score was calculated as the sum of all answered items divided by the number of answered items. Lower scores indicate poor HRQoL [13].
Physical Activity Level: The Rapid Assessment of Physical Activity (RAPA) was used to determine participants’ physical activity levels. The RAPA includes seven yes/no questions. The sum of “yes” responses indicates physical activity frequency, and intensity. The second section of the RAPA includes questions about the performance of resistance training and flexibility exercise. The RAPA has been reported to have good sensitivity (100%) and specificity (75%) for determining physical activity level. Positive and negative predictive value for determining physical activity levels were 94.4% and 100% respectively [14].
Depressive Symptoms: The Personal Health Questionnaire (PHQ-8) was used to assess prevalence and severity of depressive symptoms occurring within the past two weeks. The scale consists of 8 items with a 4-point rating ranging from 0 (not at all) to 3 (nearly every day). A score of ≥10 indicates major depression and ≥20 indicates severe major depression. Construct validity was reported at 0.75 and internal reliability was reported at 0.81 [15].
Fitness Testing
Modified Bruce Treadmill Test: The modified Bruce treadmill test was used to estimate maximal oxygen consumption (VO2 max) or cardiorespiratory fitness, as this measure is related to functional capacity and serves as a strong independent predictor of all-cause and disease-specific mortality. The modified version was chosen to ensure safety and comfort when assessing sedentary or physically inactive participants and due to availability and cost of a more direct measure. After a prolonged warm-up (6 minutes), the protocol is divided into 3-minute stages, each with a faster speed and steeper grade. The participant’s heart rate and perceived exertion were monitored throughout the test. The final score was calculated using the total time spent in working phases with the formula [(4.38 x T) - 3.9 = VO2 max]. The estimated VO2 max was then compared to gender- and age-based normative data. The modified Bruce treadmill test was chosen as the safest alternative for any sedentary participants. For the modified Bruce treadmill test, a Pearson product moment correlation coefficient between predicted and measured VO2 max of 0.94 for women without cardiac conditions (n = 5509). The average predicted error was -0.6 mL*kg-1*min-1 for the general equation [16].
Modified Push-Up Test: Using the American College of Sports Medicine (ACSM) protocol, the modified push-up test was used to estimate upper body muscular fitness. This test was chosen as a safer alternative than one-rep-max tests for sedentary or physically inactive participants and due to the availability of normative data. The test involves performing push-ups in a modified position (knees bent with lower legs in contact with the mat). The score is the total number of modified push-ups performed with proper form in one minute. The score was compared to gender- and agebased normative data. The norm-referenced test-retest reliability estimate using intraclass correlations from one-way analysis of variance (ANOVA) was R = 0.99 [16].
Curl-Up Test: Using ACSM protocol, the curl-up test was used to estimate abdominal muscular fitness. This test was chosen as a safer alternative than one-rep-max tests for sedentary or physically inactive participants and due to the availability of normative data. In a supine position, the participant repetitively lifts and lowers the upper body to a metronome set at 40 beats/minute. The score is the number of curl-ups performed with proper form to cadence. The score was compared to gender- and age-based normative data. Psychometric findings included high test-retest reliability (r = 0.98), moderately high inter-apparatus reliability (r = 0.71), and moderately high inter-tester reliability (r = 0.76) [16].
Sit-and-Reach Test: Using ACSM protocol, the sit-and-reach test was used to assess hamstring flexibility. The test was chosen because it assesses the flexibility of several muscle groups with one movement and due to the availability of normative data. This measure was chosen because hamstring flexibility is related to low back and knee joint health. Participants sat against a wall with feet placed at the 15-inch mark on a measuring tape. Keeping knees straight and feet plantarflexed, participants reached forward with both hands. The score was the farthest distance reached of three trials. The score was compared to gender-and age-based normative data. The sit-and-reach test has shown a moderate mean criterionrelated validity for estimating hamstring extensibility (r = 0.46- 0.67) [16].
Statistical Analyses
Analyses were conducted using IBM SPSS Statistics for Windows, version 27.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics (frequencies or mean ± standard deviations) were computed for each measure. Given the small sample, assumptions of normality were not met. Thus, Spearman correlations were conducted. The data that support the findings of this study are available from the corresponding author upon reasonable request.
The feasibility of conducting health and fitness assessments of women with PCOS was measured using recruitment and retention rates and participant feedback. Eighteen women answered the call for participants, with 15 meeting eligibility requirements for a recruitment rate of 83%. Fifteen women entered the study and successfully completed all phases for a retention rate of 100%. The process for each participant progressed as planned and there were no adverse events. All participants expressed satisfaction with the study and seven participants (47%) requested their laboratory and fitness results. No recommendations were made for improving the process.
“The process was easy. I wouldn’t change a thing.” (P5)
“I have never had a fitness assessment. That was fun, but harder than I thought.” (P15)
“I would like to do this again.” (P7)
“This is exciting. I hope you keep doing it and help us with PCOS.” (P1)
“Thank you for doing this for PCOS.” (P12)
“Can you tell me all my results?” (P4)
Participants (n=15) were 25.9 (±6.2) years of age and mostly White (80%). Almost half (47%) had ≥1 comorbid conditions. Characteristics of the study sample are listed in Table 1. Phenotypically, the women were obese (BMI 32.2kg/m2 ± 8.3, bodyfat 41.1% ± 8.1) with high levels of free testosterone (7.6pg/ mL ± 4.3) (Table 2). Many (53%) of the women reported regular physically activity, but not for the recommended 150 minutes/ week of moderate-to-vigorous activity. Six (40%) participants reported performing resistance training. Nine (60%) participants reported performing flexibility exercises. Table 2 presents clinical ranges, means (±SD) of the total population (i.e., reference group), a descriptor of sample means for each study characteristic, and the percent of the sample at risk for a poor health outcome based on that characteristic. Participants reported a neutral HRQoL total (3.3 ± 0.7) with low scores in the obesity/menstrual and hirsutism domains. The sample mean for depressive symptoms indicated few depressive symptoms; however, four (27%) participants scored high (≥10), indicating major depression (Table 3). Correlational analyses yielded nine statistically significant (p<.05) associations and seven associations with large effect sizes that approached statistical significance (p=0.05- 0.15) (Table 4).
| Characteristics | Frequency |
|---|---|
| Age | 25.9 ± 6.2 |
| Ethnicity | |
| African American or Black | 0% (0) |
| Hispanic, Latino/a, or Spanish Origin | 13.3% (2) |
| White | 80% (12) |
| Mix of Two | 6.7% (1) |
| Educational Attainment | |
| High School or GED | 13.3% (2) |
| Some College | 36.4% (4) |
| Bachelors | 33.3% (5) |
| Masters | 26.7% (4) |
| Employment Status | |
| Not Working | 13.3% (2) |
| Part Time | 20% (3) |
| Full Time | 66.7% (10) |
| Medical Insurance | |
| Yes | 100% (15) |
| Marital Status | |
| Single | 60% (9) |
| Married or Partnership | 40% (6) |
Table 1: Descriptive statistics of study sample (n=15).
| Variable | Mean ± SD |
|---|---|
| HRQoL Total | 3.3 ± 0.7 |
| Psychosocial/Emotional | 3.2 ± 0.7 |
| Fertility | 3.9 ± 0.9 |
| Sexual Function | 3.3 ± 0.9 |
| Obesity/Menstrual | 3.1 ± 0.9 |
| Hirsutism | 3.1 ± 1.4 |
| Coping | 3.4 ± 0.8 |
| Depressive Symptoms Total | 7.3 ± 5.3 |
Table 2: Means and standard deviations of the HRQoL and depressive symptoms survey data (n= 15).
| Variable | Reference Values | Mean ± SD | Descriptor | Sample # at Risk |
|---|---|---|---|---|
| Body Mass Index (BMI) | <18.5 (underweight) | 32.2 ± 8.3 | obese | 12 (80%) |
| 18.5–24.9 (normal) | ||||
| 25–29.9 (overweight) | ||||
| ≥ 30 (obesity) | ||||
| Waist-to-Hip Ratio (WtH) | ≤ 0.80 (low risk) | 0.81 ± 0.10 | moderate risk | 7 (47%) |
| 0.81-0.84 (moderate risk) | ||||
| ≥ 0.85 (high risk) | ||||
| Percent Bodyfat | 21 - 32% | 41.1 ± 8.1 | obese | 14 (93%) |
| Free Testosterone | 0.7-3.6 pg/mL | 7.6 ± 4.3 | high | 11 (73%) |
| Sex Hormone Binding Globulin (SHBG) | 18 - 144 nmol/L | 23.0 ± 12.9 | normal | 6 (40%) |
| High Density Lipoprotein (HDL) | 40-59 mg/dL | 63.2 ± 12.9 | high | 3 (20%) |
| Low Density Lipoprotein (LDL) | < 100 mg/dL | 91.0 ± 24.0 | normal | 5 (33%) |
| Triglycerides (TGs) | < 150mg/dL | 93.0 ± 32.0 | normal | 1 (7%) |
| VO2 Max (mL/mg/min) | Based on age | 36.8 ± 10.6 | fair | 5 (33%) |
| Upper Body Muscular Endurance | Based on age | 15.4 ± 4.0 | below average | 7 (47%) |
| Abdominal Muscular Endurance | Based on age | 21.3 ± 18.2 | below average | 12 (80%) |
| Flexibility (inches) | Based on age | 18.8 ± 2.8 | average | 6 (40%) |
Table 3: Means and standard deviations of laboratory and fitness variables compared to clinical guidelines (n=15).
| Variable | VO2 Max | Strength 1 | Strength 2 | Flexibility | RAPA 1 | RAPA 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (upper) | (abdominal) | (PA level) | (RT) | |||||||||
| R | p | R | p | R | p | R | p | R | p | R | p | |
| BMI | -0.49 | 0.07 | -0.29 | 0.29 | -0.66 | 0.01 | 0.4 | 0.14 | -0.63 | 0.12 | -0.06 | 0.82 |
| % Bodyfat | -0.59 | 0.02 | -0.44 | 0.1 | -0.61 | 0.12 | 0.22 | 0.43 | -0.72 | 0 | -0.09 | 0.74 |
| WtH | -0.06 | 0.83 | -0.05 | 0.86 | -0.28 | 0.32 | 0.15 | 0.6 | -0.47 | 0.08 | 0.25 | 0.36 |
| TestF | 0.39 | 0.16 | 0.04 | 0.89 | -0.23 | 0.39 | 0.07 | 0.82 | 0.02 | 0.95 | -0.17 | 0.54 |
| SHBG | 0.73 | 0 | 0.26 | 0.34 | 0.33 | 0.23 | 0 | 0.99 | 0.2 | 0.47 | 0.1 | 0.74 |
| HDL | 0.16 | 0.57 | 0.14 | 0.63 | 0.59 | 0.02 | -0.48 | 0.07 | -0.02 | 0.95 | -0.46 | 0.08 |
| LDL | -0.03 | 0.91 | 0.12 | 0.67 | -0.2 | 0.49 | 0.08 | 0.77 | 0.33 | 0.23 | -0.52 | 0.05 |
| TGs | -0.43 | 0.11 | -0.11 | 0.69 | -0.14 | 0.62 | 0.09 | 0.75 | -0.47 | 0.08 | -0.17 | 0.54 |
| HRQoL | 0.72 | 0 | 0.36 | 0.19 | 0.36 | 0.62 | -0.42 | 0.12 | 0.28 | 0.32 | 0.13 | 0.66 |
| DepSx | -0.67 | 0 | -0.31 | 0.25 | -0.2 | 0.48 | -0.06 | 0.84 | -0.39 | 0.15 | -0.49 | 0.06 |
Table 4: Bivariate analysis of anthropometrics, PCOS biomarkers, HRQoL, and depressive symptoms with fitness variables (n=15).
The purposes of this pilot study were to assess the feasibility of collecting health and fitness information from women with PCOS and to explore possible associations between anthropometrics, PCOS biomarkers, HRQoL, and depressive symptoms with that of fitness and self-reported physical activity levels among women with PCOS.
Feasibility
According to a review of funded and published randomized controlled trials, successful recruitment is ≥75% and successful retention is ≥80%. Using these criteria, collecting health and fitness information from women with PCOS is a feasible research approach. According to participant feedback, women with PCOS are seeking answers about their condition and are eager to participant in PCOS research [17].
VO2 Max
VO2 max is an indicator of long-term morbidity and mortality and has been recommended by the American Heart Association to be regularly assessed and used as a clinical vital sign. The finding that participants in this study had only “fair” VO2 max was alarming, as VO2 max gradually decreases 1-2% per year at ≥26 years. The mean age of this sample was 25.9 (± 6.2) years. The mean VO2 max was lower than the VO2 max reported for apparently healthy women in the same age group. However, like women in general, the participants did not meet recommended physical activity guidelines. More research is necessary to delineate factors associated with decreased VO2 max among women with PCOS [18].
Percent Body fat: Consistent with studies in other populations, VO2 max had a strong negative relationship with percent body fat (R=-0.59, p=0.02). Debate exists about the causal direction between VO2 max and body composition. Findings from a crosssectional study by Maciejczyk and colleagues (2017) suggested that high body mass, independent of cause (high lean mass or high body fat percentage) decreases VO2 max relative to total body mass. Whereas a prospective randomized trial conducted by Skrypnik and colleagues (2015) concluded that exercise increases VO2 max, which affects body composition. These studies included obese women, but not women diagnosed with PCOS [19,20].
Sex Hormone Binding Globulin (SHBG): VO2 max had a strong, positive association with SHBG (R=0.73, p<0.001). SHBG production is regulated by androgens (decrease), estrogens (increase), and insulin (decrease). The participants in this study presented with low levels of SHBG, which is often related to hyperandrogenism (also present in the participants in this study). The association between physical activity and cardiovascular fitness might be mediated, in part, through androgens; however, this has only been studied in men [21]. Findings from a systematic review about exercise effects on hormones suggest that SHBG was largely unaffected by aerobic exercise but increased with either the isolated use of resistance training or the combination of cardiovascular and resistance exercise. Additionally, women with PCOS who exercised at vigorous intensities had higher levels of SHBG than women who exercised at lower intensities [22].
Health Related Quality of Life (HRQoL): VO2 max had a strong, positive relationship with HRQoL (R=-0.72, p<0.01). Several studies have shown that women with PCOS have reduced HRQoL, especially in the psychosocial domain. However, only three randomized controlled exercise trials included HRQoL, all which revealed improved physical functioning, general health, social functioning, and mental health. Therefore, by improving VO2 max with exercise, women with PCOS may improve their HRQoL [23].
Depressive Symptoms: VO2 max had a strong, negative association with depressive symptoms (R=-0.67, p=0.01). VO2 max as a predictor of depressive symptoms has not been extensively studied among the general population and not at all among women with PCOS. In a cross-sectional study of women without PCOS (aged 26-43) who did not meet physical activity guidelines, VO2 max was found to be the only physiologic characteristic (as compared to body fat percentage, autonomic indices, and physical activity levels) to independently predict depressive symptom scores [24].
Abdominal Strength
Study participants had “below average” muscular fitness for age, a risk factor for musculoskeletal injuries and osteoarthritis due to poor posture and instability [25]. Evidence suggests that obese individuals have reduced maximum muscle strength relative to body mass compared to non-obese persons [26]. In this study, the three participants with a “normal” BMI scored average to above-average on both muscular fitness tests. The two participants with the highest BMIs and percent body fat levels were unable to perform the curl-up test and scored poorly on the push-up test. More research is necessary to determine if body fat percentage is related to muscle activation and/or muscle morphology, such as muscle volume and architecture [27].
Body Mass Index (BMI): The relationship between abdominal fitness and obesity indices among women in general is not well studied. A cross-sectional study of healthy men and women aged 18-30 found that women had lower abdominal strength due to anatomical differences and higher bodyfat mass [28]. Findings from another cross-sectional study indicated that obesity had a significant negative impact on core muscular endurance among young adult men, regardless of which obesity measure was assessed; however, a prospective study comparing normal-weight women with PCOS (phenotype D) to women without PCOS, using obesity indices, revealed that women with PCOS had lower muscle endurance than the control group of normal-weight women [29]. The researchers hypothesized that factors such as insulin resistance and visceral adiposity may damage muscle function in women with PCOS by slowing protein synthesis [30].
High Density Lipoprotein (HDL): Contrary to other research, the women in this study had high HDL, a protective factor for cardiovascular disease. The finding of high HDL was counterintuitive, given the fair VO2 max and high body fat percentages. The high HDL may be due to unique characteristics of this small sample. More research is needed with a larger sample [31].
Physical Activity Level
Physical activity level had strong negative associations with percent bodyfat among the women with PCOS in this pilot study. Physical activity is defined as any bodily movement produced by skeletal muscles that requires energy expenditure, and thus includes all types of exercise [32].
Percent Body fat: The strong negative association between physical activity level and percent body fat is consistent with the literature. Many studies have revealed that performing regular aerobic exercise, especially of vigorous intensity, decreases body fat percentage over time [33]. This was true among women with and without PCOS [34]. Performing other modalities of physical activity, such as resistance training, also reduces percent body fat over time, especially at higher intensities and in presence of greater lean mass [35].
Resistance Training Level
The performance of resistance training, as determined by RAPA, had a strong negative relationship with LDL.
Low Density Lipoprotein (LDL): The negative association between resistance training and LDL is supported by the scientific literature. Research findings of a systematic review revealed that LDL decreased with resistance training, especially when the resistance training involved either an increased volume of movement (i.e., an increased numbers of sets and/or repetitions) or a larger load (i.e., weight lifted X repetitions X number of sets). All studies examining the differential effects of exercise types showed that resistance training had a greater impact on LDL (decrease) than either fewer sets or the isolated use of aerobic activity [36]. Other researchers reported that as little as one hour per week of high-volume resistance training, independent of aerobic activity, conferred a 40- 70% lower risk for cardiovascular disease [37,38].
This pilot study was conducted to assess the feasibility of conducting health and fitness assessments of women with PCOS. Possible associations between anthropometrics, PCOS biomarkers, HRQoL, and depressive symptoms among women with PCOS with that of fitness and self-reported physical activity levels were also explored. The study involved a convenience sample, thus minimizing external validity. The sample was small; however, there were several strong statistically significant associations between participants’ fitness levels and their bio psychosocial characteristics. A glucose tolerance test was not included in this study due to cost. However, an SHBG level, a marker of insulin resistance, and consequently, a predictor of diabetes were included. Indirect fitness measures may result in less precision; however, indirect measures were chosen as safer alternatives for those women with PCOS who were sedentary or not regularly physically active. Lastly, information about medication-use was not collected and may have confounded results.
Strengths
This pilot study had several strengths. Recruitment and process procedures flowed such that 15 women with PCOS were recruited within three summer months on a college campus and uneventful study sessions. The participants provided a medically confirmed PCOS diagnosis. Anthropometrics were precisely measured by trained professionals using established protocols and body composition was determined with DEXA, a gold standard for total body composition assessment. The list of PCOS biomarkers was not exhaustive but representative and it allowed for exploration of associations between fitness and health outcomes.
In summary, collecting health and fitness data from women with PCOS is feasible. Data from fitness assessments may help identify PCOS clinical features, and thus help reveal possible health risks among women with PCOS. Research evidence, although limited at this time, supports associations between fitness and health outcomes in other populations. The scientific literature also includes evidence that most types of physical activity confer hormonal or metabolic benefit when performed regularly and at higher intensities. This study supported current research and added associations between fitness indices and health outcomes not yet explored. Randomized controlled trials in which health and fitness data are collected from women with PCOS are needed to confirm possible associations between fitness and PCOS clinical features.
The authors thank the women who participated in the study and appreciate the NIH National Institute of Nursing Research and the Center for Advancing Chronic Care Outcomes through Research and innovation (ACORN) in the College of Nursing at the University of South Carolina (UofSC) for support. The authors also thankful for the staff at the Clinical Exercise Research Center at the U of SC Arnold School of Public Health and for the laboratory services at Lexington Medical Center.
The authors declare no competing financial interests or potential conflicts of interest concerning the research, authorship, and publication of this article.
PJW conceived of the idea. PJW and CFC designed the study. PJW conducted the study with input from CFC and BMP. PJW collected the data. PJW analyzed the data with input from MDW. PJW interpreted the data with input from all authors. PJW wrote the manuscript and all authors provided feedback.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Citation: Wright PJ (2022) Fitness Assessments of Women with Polycystic Ovary Syndrome and Indications: A Pilot Study. J Women's Health Care 11(5):580.
Received: 27-Apr-2022, Manuscript No. JWH-22-16435; Editor assigned: 29-May-2022, Pre QC No. JWH-22-16435(PQ); Reviewed: 13-Apr-2022, QC No. JWH-22-16435; Revised: 25-Apr-2022, Manuscript No. JWH-22-16435(R); Published: 03-May-2022 , DOI: 10.35248/2167-0420.22.11.580
Copyright: © 2022 Wright PJ. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.