Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2020)Volume 9, Issue 2
Basil (Ocimum basilicum L.) is cultivated in many countries as an aromatic herb because of its essential oils that have many uses. In our study, nitrogen application at three levels and a tester was used, and effects on quantity and essential oil characteristics were recorded. The purpose was to explore nitrogen effects on essential oil of basil in Greece. Two commercial cultivars of basil were used: Red Rubin and Lettuce Leaf. The experiment conducted was a replicated fully randomized complete block split-split plot with the two varieties as the main factor, split factor was nitrogen levels (four rates) and split in time factor was sampling time (six dates). Nine characteristics were studied (chlorophyll content measurements-CCM, foliar surface, plant height, fresh weight, dry weight, leaves/stems ratio for fresh weight, leaves/stems ratio for dry weight, essential oil content of flowers and essential oil content of leaves). Basil plants were generally favoured by nitrogen application and many characteristics were improved. Especially for foliar surface, chlorophyll content, plant height and biomass weight (for both cultivars). Red Rubin exploited better nitrogen application in comparison to Lettuce leaf, especially for the second level. On the opposite, Lettuce leaf showed better essential oil production. The two cultivars differed also in the composition of essential oils with impact on essential oil properties. Finally, the biological cycle was accelerated for both cultivars because of increased metabolism after nitrogen application. Genotype × environment interaction was present in many cases, except for foliar surface, leaves/stem ratio and essential oil content of leaves.
Nitrogen; Essential oils; Quality; Productivity
Basil (Ocimum basilicum L.) is cultivated in many countries all over the world as an aromatic and medicinal plant and is one of the major essential oil producing species. Essential oils of basil and other aromatic plants have been used for many purposes [1-3]. The essential oil is accumulated in leaves and flowers and its high economic value is attributed to phenyl propanoids and terpenoids (and their oxygenized analogues) components [4,5]. Its aroma and other properties is attributed to the complex compositions of its main components like 1,8-cineole, methyl cinnamate, methyl chavicol and linalool [6]. In Greece, it is believed that basil is cultivated since the ancient years (B.C.) and Alexander the Great brought the plant from Middle East [7]. Sweet basil in Greece var. Genovese is the main European type cultivated also in Greece, with a typical chemical composition of linalool and methyl chavicol in a proportion of about 3 to 1, always depended on environmental conditions [8,9].
Fresh and dry herb of basil yield and essential oil content (%) and oil yield is usually increased under nitrogen fertilizer application [10] enhancing also the total biomass [11,12]. In northern countries the optimum of fertilizer application is very low because of slower development of the plant due to the colder conditions [13]. In similar studies Chatzopoulou et al. [14] referred that essential oil composition and yield were depended mainly on the specific cultivar response and not on the level of nitrogen application.
Although nitrogen fertilization is essential for crop production, its importance on the plant’s physiology, oil gland morphology, essential oil content and composition is not satisfactory studied, especially in Greek conditions [5]. In our study, nitrogen fertilizer application is considered and analysed as the main factor in relation to the morphology, physiology and essential oils production and composition of the basil plants, under the Greek Mediterraneantype environment.
General experimental procedure
The experiment was conducted in pots of 2 l (dm3), filled with local soil of Florina, Greece (SL: 59% sand, 28% silt, 13% clay, pH 6.25, organic matter 1.3%), peat and pearlite in a proportion of 1:1:1 by volume. Two commercial cultivars of basil were used: Red Rubin and Lettuce Leaf. The planting was made in 17 of February in miniplugs-256 (Herkupac). At 27 of March seedlings were transplanted in the pots. Basic fertilization was applied in 4 of April with P and K (KH2PO4) at a rate of 0.4 g.dm-3 of growing medium. A complex of micronutrients was applied (in g.dm-3 of growing medium in the pots): 0.4 Fe (EDTA), 0.15 B, 0.4 Zn (EDTA), 0.01 Mo, 0.3 Mn (EDTA), 0.22 MgO, 0.05 Cu (EDTA), 0.13 S. Nitrogen fertilizer was applied at four levels (rates): 0, 0.1, 0.2, 0.4 (in g.dm-3 of growing medium in the pots) as ammonium sulfate (21% N), at 12 of April.
The basil plants were irrigated with the same amount of water, as necessary, every 2 days for 2 min. The experiment was conducted under controlled conditions; no presence of diseases or pests was found, and thus, no chemical protection was used. The experiment conducted was a replicated fully randomized complete block splitsplit plot (in time) design with the two varieties as the main factor, split factor was nitrogen levels (four rates) and split in time factor was sampling time (six dates: 33, 45, 62, 79, 100 and 117 days from nitrogen application).
Measurements
Chlorophyll Content Measurements (CCM): It was measured by a portable instrument (CCM 200, Opti-Sciences, Tyngsboro, MA). Ten measurements per plot were performed on leaves of the same developmental stage and similar size. This instrument uses differential transmission to determine the absorbance of chlorophyll pigments [15]. Beam was standardized according to the manufacturer’s instructions. Beams are generated by LEDs and leaf tissues and pigments are unharmed. The region measured by fully covers basilicum leaves which are almost at the same size.
Foliar surface: it was measured in cm2 in all stages (six dates: 33, 45, 62, 79, 100 and 117 days from nitrogen application) by the instrument Portable Area Meter model LI-3000 (Li-Cor, Inc. N.E. USA), after proper preparation of the plants. Ten leaves were used for measurements. Plant height in cm: it was measured in six stages (six dates: 33, 45, 62, 79, 100 and 117 days from nitrogen application) from 10 plants. A conventional ruler was used in all stages of plant development.
Fresh weight: it was measured in a balance (in g) in all stages (six dates: 33, 45, 62, 79, 100 and 117 days from nitrogen application), and ten plants (replications) was used. The plants were harvested at the full flowering (117 days) by cutting off the above-ground portion of the stem above its lignified parts.
Dry weight: Drying followed in a specific oven at 72°C, until stabilization of weight, and dry weight was measured by a high precision balance (in g).
Leaves and stems weight: The ratio between leaves weight and stems weight was calculated separately (for fresh and dry weight).
Essential oils: Cut herbs were dried in a drying oven at a temperature of 35°C. The essential oil was extracted from air-dried powdered material of flowers (100 g) of three different dates (57, 82 and 121 days from fertilizer application) and of leaves at the same dates (300 g). They filled a glass Clevenger-type distillation apparatus and the material had undergone hydro-distillation for 3 h. The assays were conducted in triplicate. The extracted essential oil was stored in an amber glass container at a temperature of -5°C until the time of chromatographic separation. Qualitative and quantitative analysis of the basil essential oil was performed using a Shimadzu GC-2010, GCMS-QP2010 (gas chromatograph with a mass detector) and a Flame Ionization Detector (FID). A temperature of 50°C was applied for 5 min, and then the temperature was incremented to 290°C at a rate of 4°C min-1. Helium was the carrier gas, with a constant flow of 1 ml min-1, injector 290°C, split 1:30. One microliter of the solution was injected. Identification of constituents was done on the basis of retention index (RI) (with reference to the homologous series of n-alkanes C8-C25, under identical experimental condition), MS library search (NIST 05.L and NIST 98.L libraries with >70% similarity and WILEY), and by comparison with MS literature data [16,17]. The retention indices were determined based on the v-alkane series.
The obtained results of the assays were statistically analyzed using analysis of variance for 2-way cross-classification and also using a factorial scheme (ANOVA) based on main factors: cultivars, nitrogen application, sampling period, in SPSS v.17 software, evaluating least significant difference (LSD) calculations at the level of significance of 0.05 [18]. Software used for mass spectral deconvolution and identification conditions was AMDIS Version. 2.1., and a MATLAB routine was also used for quantifying data [19].
The studied characteristics were differentiated statistically under different nitrogen levels as shown in ANOVA presented in Table 1. Generally, increased nitrogen rate resulted in greater CCM values (chlorophyll content measurements), foliar surface, plant height, fresh weight, dry weight and essential oil content of leaves (Figures 1-6). Leaves/stems ratio for fresh weight, leaves/ stems ratio for dry weight and essential oil content of flowers showed a decrease, which was fluctuating in the last case (Figures 7-9). Foliar surface, plant height and dry weight are presented in more details in relation to sampling time in Figures 10-12. Both cultivars used (Lettuce leaf and Red Rubin) showed statistically significant differences for all characteristics as shown in Table 1 and evident differences for the main components of the their essential oils (Table 2) as reported also by other researchers [8,20,21]. Generally from our findings, nitrogen application accelerated plant metabolism and biological cycle and this resulted in faster flowering of plants. In the case of essential oil content of flowers the two cultivars showed different behavior and significant Cultivar-Nitrogen interaction was present (Table 1). In the second sampling period Red Rubin showed a negative correspondence to nitrogen application for essential oil content of flowers and this is reflected in Cultivar-Sampling and Nitrogen-Sampling interactions in Table 1, also presented graphically in Figure 9. For Lettuce leaf, there was found an increased value in the first sampling, but a slight decrease in the second sampling period (Figure 9). Triple interaction was found significant for plant height and dry weight and many double factor interactions were found for CCM, plant height and essential oil content of flowers (Table 1). This finding indicates that according to cultivation techniques we may have different results on main characteristics studied. It is reported that harvest time or sampling time, or even plant stage affects some characteristics like chlorophyll content or other biomass and quality characteristics [22,23]. Generally, changes in essential oil composition are expected in different conditions and may result in reduced activity and effectiveness [24,25]. Summarizing there was found ten interactions, indicating a generalized Genotype × Environment (GXE) interaction. Thus, genotype × environment interaction was found significant, except for foliar surface, leaves/ stem ratio and essential oil content of leaves.
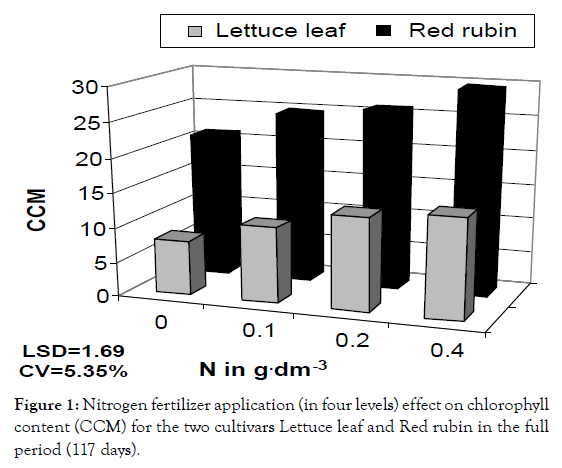
Figure 1: Nitrogen fertilizer application (in four levels) effect on chlorophyll content (CCM) for the two cultivars Lettuce leaf and Red rubin in the full period (117 days).
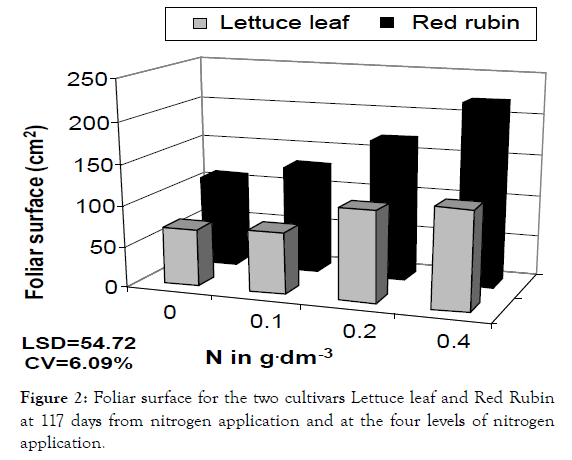
Figure 2: Foliar surface for the two cultivars Lettuce leaf and Red Rubin at 117 days from nitrogen application and at the four levels of nitrogen application.
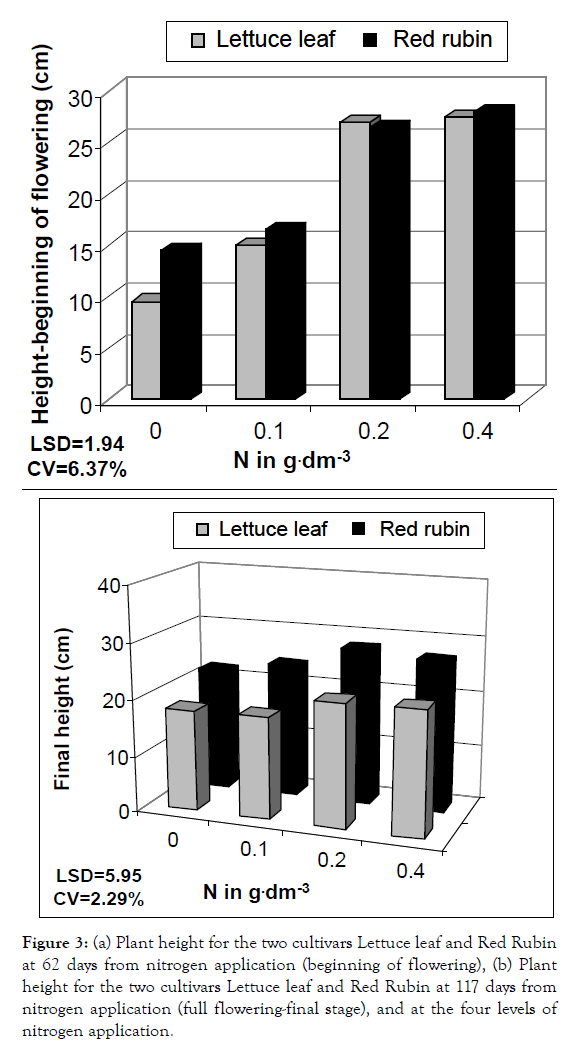
Figure 3: (a) Plant height for the two cultivars Lettuce leaf and Red Rubin at 62 days from nitrogen application (beginning of flowering), (b) Plant height for the two cultivars Lettuce leaf and Red Rubin at 117 days from nitrogen application (full flowering-final stage), and at the four levels of nitrogen application.
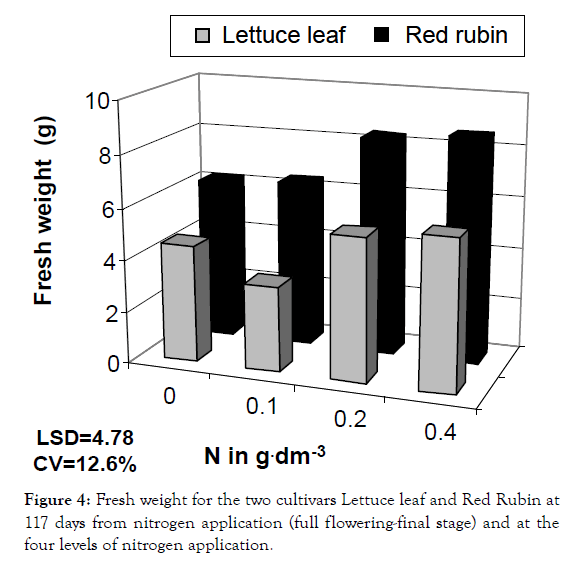
Figure 4: Fresh weight for the two cultivars Lettuce leaf and Red Rubin at 117 days from nitrogen application (full flowering-final stage) and at the four levels of nitrogen application.
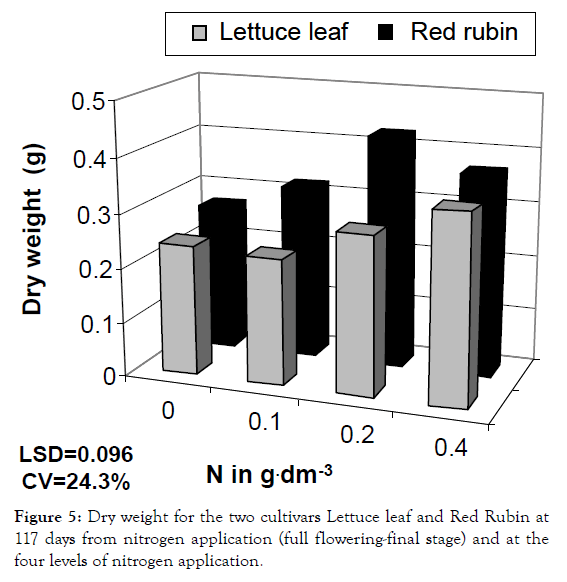
Figure 5: Dry weight for the two cultivars Lettuce leaf and Red Rubin at 117 days from nitrogen application (full flowering-final stage) and at the four levels of nitrogen application.
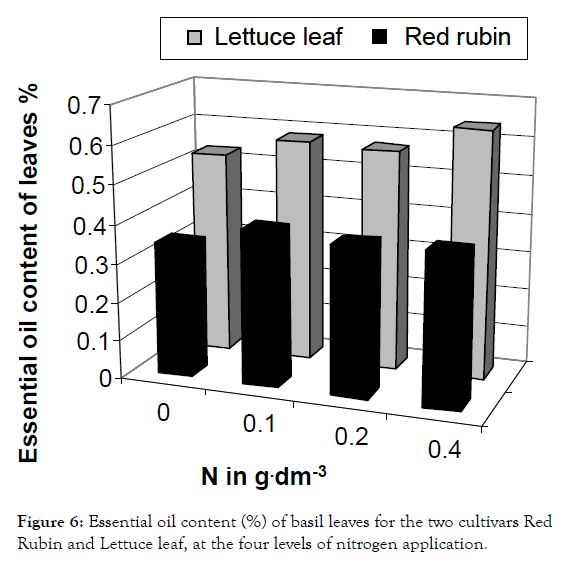
Figure 6: Essential oil content (%) of basil leaves for the two cultivars Red Rubin and Lettuce leaf, at the four levels of nitrogen application.
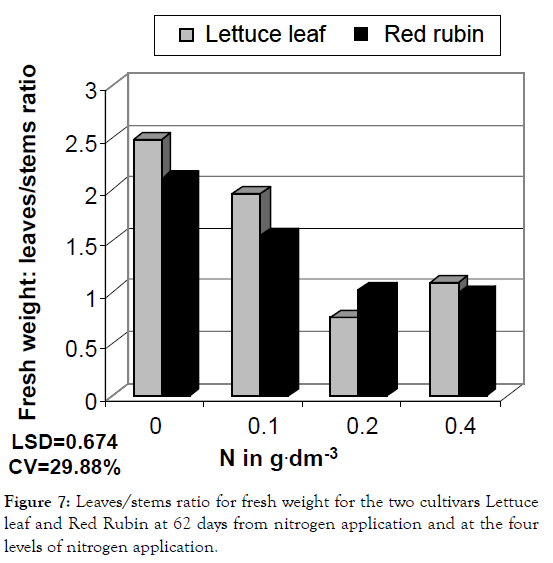
Figure 7: Leaves/stems ratio for fresh weight for the two cultivars Lettuce leaf and Red Rubin at 62 days from nitrogen application and at the four levels of nitrogen application.
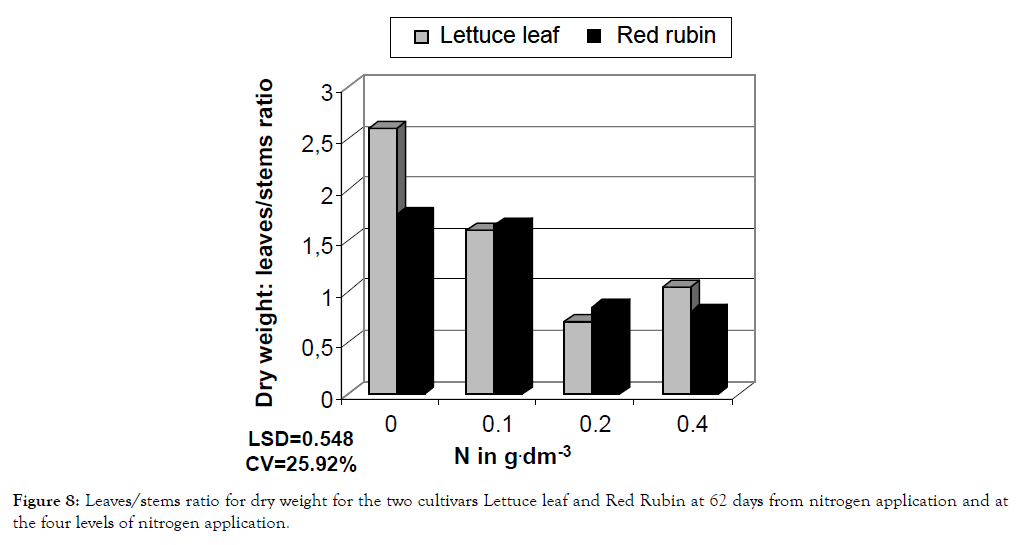
Figure 8: Leaves/stems ratio for dry weight for the two cultivars Lettuce leaf and Red Rubin at 62 days from nitrogen application and at the four levels of nitrogen application.
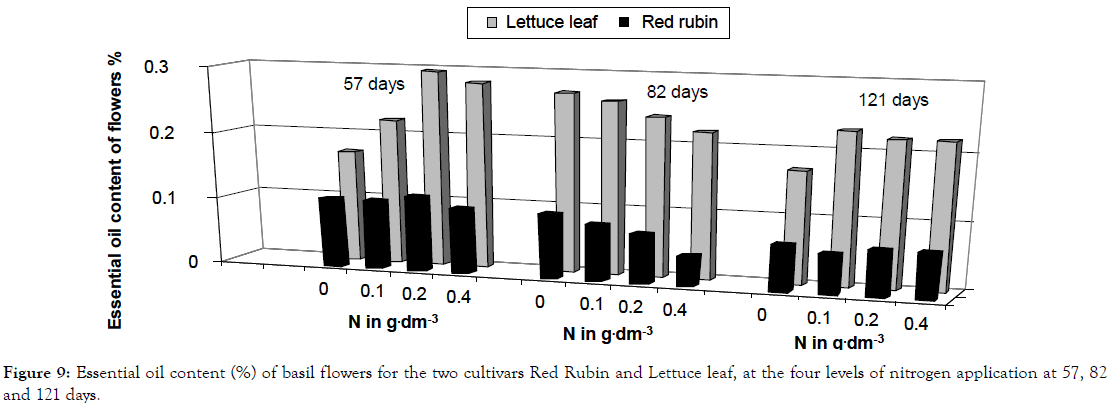
Figure 9: Essential oil content (%) of basil flowers for the two cultivars Red Rubin and Lettuce leaf, at the four levels of nitrogen application at 57, 82 and 121 days.
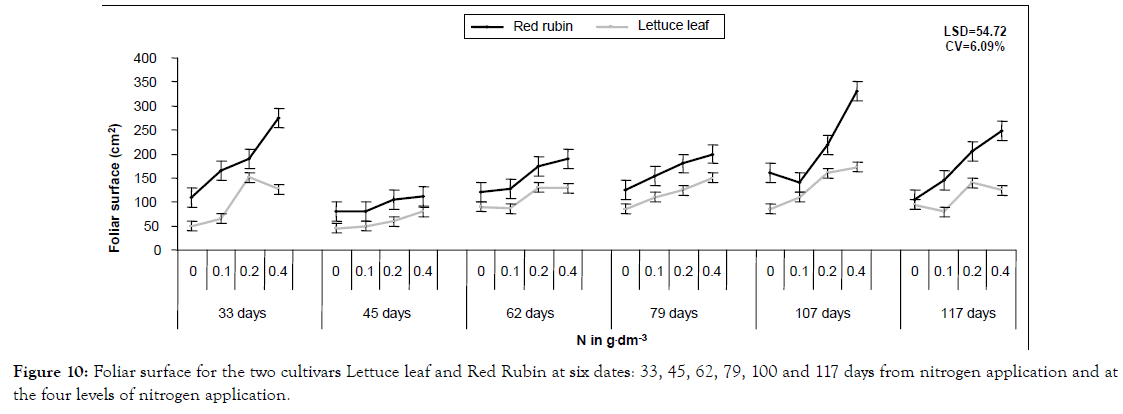
Figure 10: Foliar surface for the two cultivars Lettuce leaf and Red Rubin at six dates: 33, 45, 62, 79, 100 and 117 days from nitrogen application and at the four levels of nitrogen application.
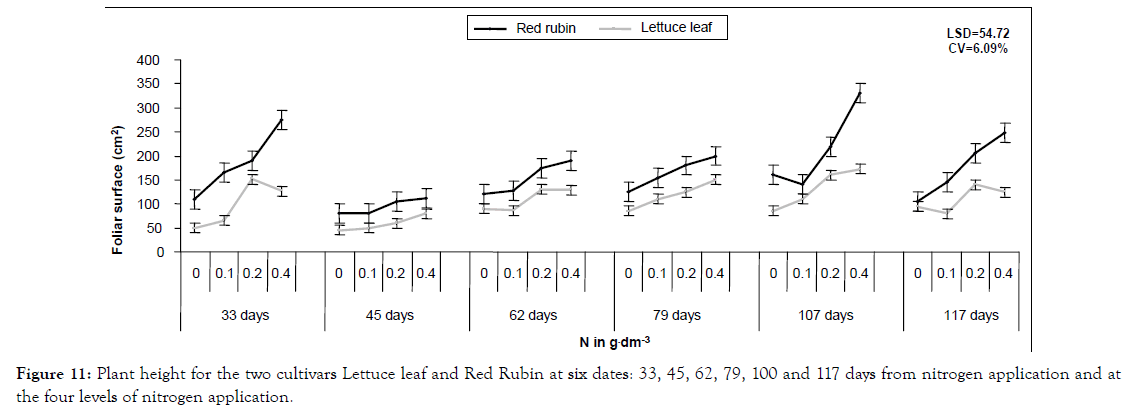
Figure 11: Plant height for the two cultivars Lettuce leaf and Red Rubin at six dates: 33, 45, 62, 79, 100 and 117 days from nitrogen application and at the four levels of nitrogen application.
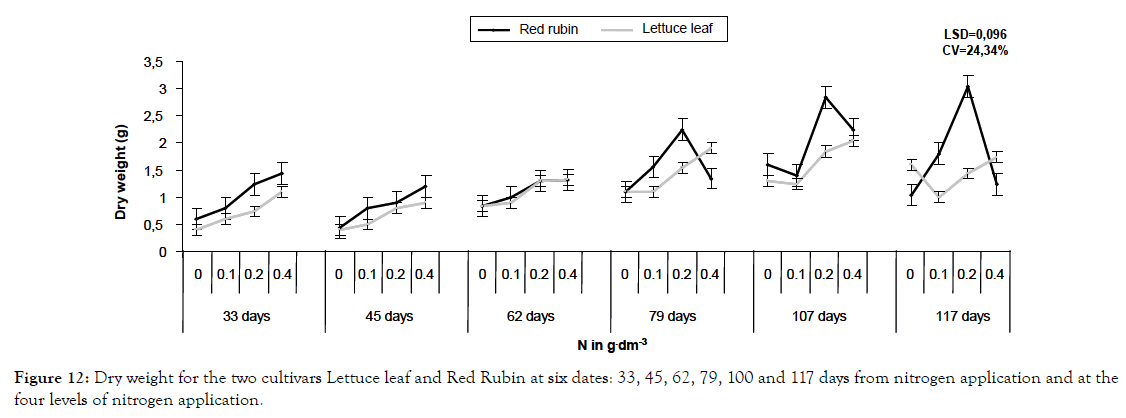
Figure 12: Dry weight for the two cultivars Lettuce leaf and Red Rubin at six dates: 33, 45, 62, 79, 100 and 117 days from nitrogen application and at the four levels of nitrogen application.
| Factors | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Replications | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Cultivars C | * | * | * | * | * | * | * | * | * |
| Nitrogen N | ** | * | * | * | * | * | * | * | * |
| Sampling S | ** | * | * | * | * | - | - | * | - |
| C × N | * | ns | * | * | * | ns | ns | * | ns |
| C × S | * | ns | * | ns | ns | - | - | * | - |
| N × S | * | ns | * | ns | ns | - | - | * | - |
| C × N × S | ns | ns | * | * | ns | - | - | ns | - |
Table 1: ANOVA significance levels for factors cultivars (C), Nitrogen levels (N) and sampling time (S) and their interactions, for the nine characteristics: (1=CCM, 2=Foliar surface, 3=Plant height, 4=Fresh weight, 5=Dry weight, 6=Leaves/stems ratio for fresh weight, 7=Leaves/stems ratio for dry weight, 8=Essential oil content of flowers, 9=Essential Oil content of leaves).
| Lettuce leaf Component, RI (Retention Index), % |
Red rubin Component, RI (Retention Index), % |
||
|---|---|---|---|
| Sabinene, 969, * | Estragol, 1198, 85.1 | Sabinene, 976, 0.3 | -- |
| Pinene, 973, 0.2 | Bornyl acetate, 1251, 0.4 | Pinene, 981, 0.2 | -- |
| Myrcene, 993, 0.3 | Eugenol, 1397, 2 | Myrcene, 993, 0.8 | Eugenol, 1397, 78.1 |
| Kineol, 1023, 2 | Bergamontene, 1440, 1.6 | Kineol, 1023, 3.6 | Bergamontene, 1440, 3.6 |
| Ocimene, 1046, 0.7 | Humulene, 1466, 0.1 | -- | Humulene, 1466, 0.4 |
| Linalool, 1097, 3 | Germacrene, 1487, 0.3 | Linalool, 1097, 7 | Germacrene, 1487, 0.6 |
| Camphor, 1146, 0.7 | Cadinene, 1547, * | -- | Cadinene, 1547, 0.1 |
| Limonene, 1033, 3 | a-Cadinol, 1670, * | Limonene, 1033, 5 | a-Cadinol, 1670, 0.1 |
Table 2: Main components (their retention index RI and their content %) content of the essential oils extracted from the two basil cultivars Lettuce leaf and Red Rubin (Leaves and flowers).
In a more detailed analysis, nitrogen application increased CCM by 41%, 64% and 81% at the three levels of nitrogen for Lettuce leaf and 165, 21% and 36% for Red Rubin, respectively (Figure 1). Foliar surface was also affected by all factors (cultivar, nitrogen and sampling time) but no interaction was found. At the highest nitrogen level foliar surface was increased by 143 and 96% for Lettuce leaf and Red Rubin respectively (Figure 10). Plant height was affected positively by nitrogen application (Figures 3 and 11) but the strong interaction of all factors (cultivar, nitrogen and sampling time) showed that this effect may depend on combination of the stage and the cultivar for better exploitation of the high nitrogen inputs. Plant height was increased by 40% as a mean of all nitrogen levels. In Figure 3a, height as a mean increased by 143% and 63% for Lettuce leaf and Red Rubin respectively, at full flowering stage. Fresh weight was increased by 128% and 123% for Lettuce leaf and Red Rubin respectively, at highest nitrogen level (Figure 4).
For dry weight, Red Rubin exhibited higher values and in the first sampling period it was increased by 214% and 140% for Lettuce leaf and Red Rubin respectively, at highest nitrogen level (Figures 5 and 12). These positive changes are attributed to increased photosynthesis and faster metabolism after nitrogen application [23,26-30], especially for LAI and increased foliar surface, this was attributed to the increased number of leaves rather than to expansion of leaves themselves [31].
The cultivar Lettuce leaf (in contrast to Red Rubin) was favored by nitrogen application and essential oil content of leaves was increased by 8, 7 and 22% respectively at the three nitrogen levels (Figure 6), as also reported by Zheljazkov et al. [32], Daneshian et al. [33] and Nurzynska-Wierdak et al. The increased essential oil production may be due to increased foliar surface after nitrogen application and the presence of numerous and larger essential oil glandular trichomes in relation to developmental stage of basil plant and environmental conditions [9,34].
Basil plants were generally favoured by nitrogen application. Especially for foliar surface, chlorophyll content, plant height and biomass weight (for both cultivars Lettuce leaf and Red Rubin). Red Rubin exploited better nitrogen application in comparison to Lettuce leaf, especially for the second level of nitrogen. On the opposite, Lettuce leaf showed better essential oil production. The two cultivars differed also in the composition of essential oils and thus, in their properties. The biological cycle was accelerated for both cultivars because of increased metabolism after nitrogen application. Genotype × environment interaction was present in many cases (ten double and triple interactions in total), except for foliar surface, leaves/stem ratio and essential oil content of leaves.
This research work is dedicated to late professor A.C. Fasoulas, who was one of the pioneers of Agronomy science and breeding in Greece. Also, to late professor Stelios Zotis for his contribution in statistical processes.
Citation: Ipsilandis CG, Greveniotis V, Deligeorgidis NP, Pampouktsi P, Tsakiropoulos I, Boudouroglou N (2020) Fertilizer Application in Basil (Ocimum basilicum) Cultivation in Greece. Med Aromat Plants (Los Angeles) 9: 345. doi: 10.35248/2167-0412.20.9.345
Received: 08-Mar-2020 Accepted: 18-Mar-2020 Published: 25-Mar-2020 , DOI: 10.35248/2167-0412.20.9.345
Copyright: © 2020 Ipsilandis CG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.