
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Short Communication - (2023)Volume 14, Issue 4
Commercial products like Exparel® and Bupisome®, which contain bupivacaine, offer prolonged, non-opioid alternatives for the management of post-operative pain. These pharmaceutical products share a common feature: the nanoformulation of the local anesthetic bupivacaine in Ionic Gradient Liposomes (IGL). IGL are specially designed liposomes known for their extensive loading capacity, which allow them to both extend the effectiveness and reduce the toxicity of weak bases such as local anesthetics. We outline here the benefits of two IGL formulations designed specifically for the encapsulation of bupivacaine in its enantiomeric excess form (S75BVC) or Etidocaine (EDC).
Bupivacaine; Etidocaine; Ionic gradient liposomes; Anesthesia
Bupivacaine and Etidocaine, belonging to the aminoamide family Local Anesthetics (LA), are long-acting agents that grapple with significant systemic toxicity issues. For instance, at the closing of the 20th century enantiomeric excess bupivacaine (S75BVC) was introduced into the market as Novabupi®, with the intention of curtailing the toxicity associated with traditional (racemic mixture) bupivacaine formulations [1]. Further, in 2008, the Food Drug Administration (FDA) discontinued the clinical use of etidocaine due to alleged toxicity issues.
Drug Delivery Systems (DDS) that support sustained release and decrease LA toxicity could provide a solution to bupivacaine's cardiotoxicity and pave the way for the reintegration of Etidocaine (EDC) into clinical practice [2]. Among various DDS options, liposomes are by far the most known [3]. However, clinicians may not be fully aware that liposomes can be prepared with an ionic gradient to enhance the loading of LA [4-7].
Ionic Gradient Liposomes (IGL), as depicted in Figure 1, have shown promise for the delivery of LA agents such as racemic bupivacaine [8-10], ropivacaine [11], and dibucaine [12]. Notably, IGL facilitated the upload of up to 2% BVC, which, upon intradermal administration to a human volunteer, provided 48 hours of anesthesia [13]. Furthermore, the utility of IGL was demonstrated by enabling the infiltrative delivery of Dibucaine–a conventionally topical LA, Yielding up to 27 hours of anesthesia in mice (Figure 1).
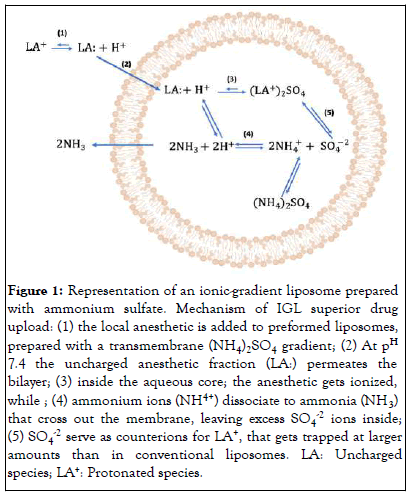
Figure 1: Representation of an ionic-gradient liposome prepared with ammonium sulfate. Mechanism of IGL superior drug upload: (1) the local anesthetic is added to preformed liposomes, prepared with a transmembrane (NH4)2SO4 gradient; (2) At pH 7.4 the uncharged anesthetic fraction (LA:) permeates the bilayer; (3) inside the aqueous core; the anesthetic gets ionized, while ; (4) ammonium ions (NH4+) dissociate to ammonia (NH3) that cross out the membrane, leaving excess SO4-2 ions inside; (5) SO4-2 serve as counterions for LA+, that gets trapped at larger amounts than in conventional liposomes. LA: Uncharged species; LA+: Protonated species.
Recently, our group employed IGL technology to encapsulate both S75BVC [14] and EDC [15]. The objective of this brief commentary is to update anesthesiologists about the benefits of IGL formulations for long-acting LA, for which systemic toxicity poses a substantial challenge.
Both IGL formulations were prepared with 250 mM (NH4)2SO4 in the inner aqueous core of phosphatidylcholine: cholesterol liposomes, optimized by Design of Experiments. The optimized Ionic Gradient Liposomes (IGLS75BVC and IGLEDC) were characterized regarding the mean diameter, polydispersity, zeta potential, and LA encapsulation efficiency. Colorimetry and magnetic resonance provided evidence of LA interaction/ diffusion trough the lipid bilayer (a necessary step for drug entrapment in IGL- (Figure 1).
IGLS75BVC and IGLEDC showed mean diameters of 310-480 nm, low size polydispersity, (<0.20), negative zeta potentials (-15 mV), and good shelf-stability (360 days at 4°C or 180 days under extreme conditions: 40°C and 75% relative humidity). The morphology of the liposomes was followed either by transmission and Cryogenic electron microscopy and revealed the well-defined contours of spherical uni and oligolamellar vesicles.
As expected, IGL promoted the sustained release of the anesthetics. In vitro, at 37°C the time for equilibrium was reached after 24 hours and 14 hours, and the kinetics followed non-Fickian distribution (Michaelis-Menten or Weibull models, for IGLS75BVC and IGLEDC, respectively).
Both IGLS75BVC and IGLEDC decreased LA intrinsic toxicity, in vitro and in vivo.
In vitro toxicity
Encapsulation into liposomes decreased the in vitro cytotoxicity of both anesthetics against neuronal (primary Schwann cells). After 24 hours, the concentration to decrease cell viability by 50% (IC50) was 1.14 mM for cells treated with free S75BVC and 2.87 mM with IGLS75BVC. As for EDC, IC50 values of 4 mM (free EDC) and 10 mM (IGLEDC) were measured.
In vivo toxicity
Biochemical and morphological analyses have shown that IGLS75BVC have minimal toxic effects in the animals, the carrier itself being able to stimulate the immune system and improve the safety of S75BVC anesthesia.
As for EDC, the C. elegans model of toxicity was used. After treatment with 0.5% EDC ca. 75% of the larvae were viable. Toxic effects were only observed at 1% and 2% (super clinical etidocaine doses) and encapsulation into IGLEDC significantly increased the survival rate of the larvae, from 70% to 92% (1%) and from 30% to 63% (2%), reinforcing the idea that IGLEDC may enable the safe use of EDC.
Antinociceptive effects
Using the PWPT, the blockage of the sciatic nerve of adult male Wistar rats was measured. 0.5% IGLS75BVC promoted 2 times longer sensory block (9 hrs) than reported for IGL formulations containing racemic bupivacaine [16,17]. Moreover 0.25% IGLS75BVC promoted twice the anesthesia duration of 0.5% freeS75BVC.
In the same experimental setup, IGLEDC improved in 40% the area under the curve (analgesia) effect versus time [18] regarding free EDC. Additionally, injection of lower doses (0.25% IGLEDC) elicited an anesthetic effect equivalent to that of 0.5% free EDC, thus suggesting an additional strategy to mitigate its purported systemic toxicity through dose reduction. For us, these results are indicative that EDC can be safely reintroduced in clinics, since its discontinuity by the FDA [19,20] was controversial, with no proofs of toxic effects directly promoted by EDC being reported [21].
IGL are very interesting DDS for local anesthetics giving their capacity to foster sustained drug release at the target site, thereby extending the duration of sensory nerve blockade. We believe IGLEDC might facilitate the safe clinical reintroduction of EDC, reviving the benefits of this long-acting local anesthetic in the management of surgical and post-surgical pain. In terms of IGLS75BVC it promoted nine hours of anesthesia, 2.25 times longer than previously observed with IGL such as Exparel® and Bupisome® after infiltrative administration. For both LA the sensorial blockades were achieved at lower doses of the liposomal formulations, pointing out IGLS75BVC and IGLEDC as promising candidates for future clinical trials.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Oliveira JD, de Lima FF, de Paula E (2023) Exploiting Ionic Gradient Liposomes for Anesthetic use: The Case of Enantiomeric-Excess Bupivacaine and Etidocaine. J Anesth Clin Res. 14:1111.
Received: 31-Jul-2023, Manuscript No. JACR-23-25935 ; Editor assigned: 02-Aug-2023, Pre QC No. JACR-23-25935 (PQ); Reviewed: 17-Aug-2023, QC No. JACR-23-25935 ; Revised: 24-Aug-2023, Manuscript No. JACR-23-25935 (R) ; Published: 31-Aug-2023 , DOI: 10.35248/2155-6148.23.14.1111
Copyright: © 2023 Oliveira JD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.