
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Research Article - (2025)Volume 16, Issue 1
Background: Post-anesthetic shivering is a common complication of anesthesia, which accounts for much discomfort in postoperative patients and may increase postoperative complications in high-risk patients. The main purpose of this review is systematically reviewing articles and finally drawing an evidence-based guideline for the use of ketamine for management of post-anesthetic shivering.
Materials and methods: Reporting Items for Practice Guidelines in Healthcare (RIGHT) process was followed in reporting the review. A literature search was conducted using the Hinari database, PubMed, Google Scholar and Cochrane review. Important words: Ketamine, anti-shivering, post-anesthetic shivering and shivering control were employed. Following the process of extracting and filtering results according to the interventions, outcome and methodological quality, two systematic reviews and meta-analyses, one observational study, sixteen randomised control trials and one outcome study were evaluated for quality. A determination was made regarding their degree of evidence and recommendation grade.
Conclusion: The majority of studies indicate that the low dose of ketamine, 0.25 mg/kg, is beneficial in treating postanesthetic shivering without resulting in any complications. However, a small number of studies suggest that, despite not causing any complications, the drug has dubious effectiveness in preventing post-anesthesia shivering following general anaesthesia and that lower doses appear to offer no benefit at all. Conversely, ketamine at a dose of 0.5 mg/kg shows greater results than a placebo and may even be just as effective as pethidine; nonetheless, adverse effects such as delirium and hallucinations have been recorded. To effectively manage post-anesthesia shivering and consequently prevent its recurrence, we advise using ketamine to treat or prevent it at doses of 0.15 mg/kg to 0.5 mg/kg.
Anesthesia; Ketamine; Hallucinations; Electrocardiography
BSAS: Bed Side Assessment of Shivering; ECG: Electrocardiogram; EEG: Electroencephalograph; EMG: Electromyogram; ENT: Ear Nose Throat; GA: General Anesthesia; GOR: Grade of Recommendation; IV: Intravenous; ICU: Intensive Care Unit; LOE: Level of Evidence; NE: Norepinephrine; NMDA: N-Methyl D Aspartate; OR: Operating Room; PACU: Post Anesthesia Care Unit; PAS: Post Anesthesia Shivering; PONV: PostOperative Nausea and Vomiting; RA: Regional Anesthesia; RCT: Random Control Trail; TTM: Therapeutic Temperature Modulation
Background
Shivering is an attempt at defense. By contracting muscles, it raises body temperature production. Shivering after surgery is now a regular anaesthesia side effect. About 50% to 65% of individuals experience it following general anaesthesia and 30% do so during regional anaesthesia. Shivering is just as common with regional anaesthesia as it is during general anaesthesia. Until the block effects are removed, patients' hypothermia persists at the block level. This is due to the fact that regional anaesthesia interferes with the lower limbs' ability to submit sensory information to nerve centres. Again, less heat is produced as a result of the paralysis of the lower limb muscles. Consequently, the duration required for the patient to regain consciousness is doubled in cases of regional anaesthesia compared to general anaesthesia [1].
The thermal signals of the occluded region mostly cold ones are impeded by spinal anaesthesia. It results from a decrease in the threshold for shivering and vascular contraction. In addition to lowering body temperature, spinal anaesthesia causes vasodilatation as a result of the sympathetic block. The patient then becomes vulnerable to shivering and hypothermia. One common anesthesia-related consequence that may potentially make pain worse is Post-Anesthetic Shivering (PAS). It is marked by involuntary movement, which might impair one or more muscle groups. It is an extremely uncomfortable and stressful physiological condition. Monitoring of oxygen saturation (SpO2 ) and Electrocardiography (ECG) may be hampered by PostAnesthetic Shivering (PAS).
More significantly, it can raise carbon dioxide production and minute ventilation while also increasing oxygen consumption. Furthermore, it's thought that post-anesthetic shivering raises death rates in heart attack patients and the elderly.
The cause of shivering is not well recognised. Post-operative shivering can be caused by both thermoregulatory and nonthermoregulatory causes, such as exposure to cold temperatures, insufficient pain management and opiate withdrawal.
There is currently no established gold standard for either prevention or treatment. Numerous pharmaceutical interventions and techniques have been employed to mitigate post-operative shivering, such as meperidine, alfentanil, tramadol, magnesium sulphate, ondansetron, olasetron and dexmedetomidine.
Ketamine plays a part in thermoregulation as a competitive antagonist of the N-Methyl-d-Aspartate (NMDA) receptor. In locus ceruleans, the NMDA receptor regulates noradrenergic and serotoninergic neurons. In addition, it directly stimulates central sympathetic nervous system and inhibits norepinephrine production.
Heat redistribution from the core to the periphery may be lessened by absorption into postganglionic sympathetic nerve terminals. By generating heat that is not brought on by vibration and acting on the hypothalamus and beta-adrenergic receptors, ketamine most likely controls shivering. Ketamine's quick start is ensured by its fast acting nature and fat solubility. Ketamine acts by diffusing into passive tissues, much like other intravenous anaesthetics do after a single intravenous dose. The primary metabolite of ketamine, nor ketamine, is eliminated in the urine and has a lower potency than ketamine.
Justification
Shivering is a significant complication of hypothermia. The most common reason for shivering is cold, but some shivering, including essential tremor, is not thermoregulatory. The processes that lead to core hypothermia in regional and general anesthesia are similar. All general anesthetics markedly impair normal autonomic thermoregulatory control.
Shivering can also be brought on by postoperative discomfort or the production of cytokines during the surgical procedure. About 50% of patients with a core temperature of 35.5â, 40% of unwarned patients recovering from general anaesthesia and 90% of patients with a core temperature of 34.5â experience shivering. Unintentional hypothermia is linked to a number of unfavourable postoperative outcomes. Significant adrenergic activity and discomfort are linked to shivering [2].
Shivering increases, the risk of unfavourable cardiac outcomes by doubling or even tripling oxygen use and carbon dioxide generation. An elevated risk of cardiac problems is also associated with a significant rise in plasma catecholamine levels. Additionally, shivering raises intracranial and intraocular pressure. Interference with blood pressure and heart rate monitoring, as well as an elevation in metabolic rate and lactic acidosis.
Many scientific studies have been conducted to support pharmacological interventions for post-anesthesia shivering; however, only a small number of these studies have included ketamine as a component that is required to close the gap in the management of post-anesthesia shivering and as an adjuvant treatment for non-pharmacologic interventions that improve post-anesthesia shivering and its consequences. Reviewing and creating guidelines for managing post-anesthesia shivering is highly advantageous for surgical patients as well as anaesthesia specialists. The purpose of this review is to develop guidelines for the management of post-anesthesia shivering in surgical patients. These guidelines will include pharmacologic interventions, such as the use of ketamine, to help reduce the number of patients who suffer from acute and chronic complications related to inadequate post-anesthesia shivering management.
Objective
The aim of this guideline is to provide anesthesia professionals with an evidence-based guide to manage post-anesthesia shivering in surgical patients by using ketamine.
Scope of the guideline
Target audience of this guideline involves anesthesia providers. All surgical patients susceptible for intra operative or postoperative shivering, except pediatrics age less than five years and patients with coexisting diseases like toxic thyroid, neuromuscular diseases, seizure/epilepsy, were the target population of this guideline. Previously the commonly practiced interventions for management of shivering in surgical patient were non-pharmacological methods through re-warming by using either active or passive warming and sometimes using pharmacological methods like opioids, etc.
This guideline will address management of post-anesthesia shivering in surgical patients by ketamine interventions supported by current best evidences. There is no international or national published guideline that specifically focused on management of post-anesthesia shivering using ketamine as far as my search [3].
The review is reported in line with the PRISMA check list criteria. A systematic and hand search of literature was done from Cochrane review, PubMed, Google Scholar and Hinari database. The search performed using key words for PubMed and Cochrane (shivering and ketamine or intra-operative shivering or postoperative shivering or anti-shivering or preventing shivering or shivering management or shivering relief or treatment) and by using full sentences search for Google scholar. Observational, interventional studies, meta-analysis and systematic review studies, full articles published from 2005 to June, 2021 and articles written in English language were included in this review.
The results of the search engine were filtered based on the interventions, outcome, data on population and methodological quality. Only articles involving post-anesthesia (intra-operative/ post-operative) shivering management in surgical populations done either under GA or RA were included, whereas articles with, background post-anesthesia shivering non-pharmacological managements and pharmacological managements other than ketamine intervention were excluded. After extraction and filtering with a patient population and exclusion criteria’s were done; 2 Systematic review and meta-analysis, 1 observational study, 16 randomized control trail and 1outcome study were appraised for quality and conclusion was made based on their level of evidence and grades of recommendations that adapted from oxford center for evidence based medicine (Table 1).
| Level of evidence | Grading criteria | Grade of recommendation |
| 1a | Systematic reviews of RCTs including meta-analysis | A |
| 1b | Individual RCT with narrow confidence interval | A |
| 1c | All or none randomized controlled trials | B |
| 2a | Systematic review of cohort study | B |
| 2b | Individual cohort including low quality RCT | |
| 2c | Outcome research study | C |
| 3a | Systematic review of case control studies | C |
| 3b | Individual case control study | C |
| 4 | Case series, poor quality cohort and case control studies | C |
| 5 | Expert opinion without explicit critical appraisal, or based on physiology, bench research or “first principles” | D |
Table 1: Level of evidences and grades of recommendations.
Plan for dissemination
This guideline will be disseminated to all operating room theatres at which surgical procedures under anesthesia are performed and to all hospitals in which surgeries are done under either general or regional anesthesia. This evidence based practice guideline will be revised within five years (Figure 1).
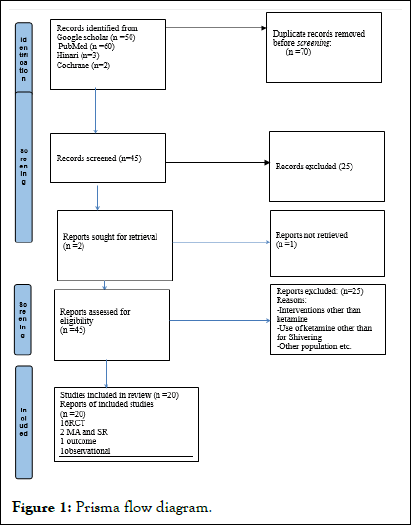
Figure 1: Prisma flow diagram.
Objective assessment of shivering via direct observation
The ability to observe things carefully will enable you to recognise changes in the skin's condition [4]. The initial sign of heat loss is pilocytic erection or goose bumps, which happen as the body tries to divert blood from the peripheral compartment. The body starts to shiver in an effort to generate heat and raise its temperature while attempting to lessen the loss of heat. Early detection and measurement of shivering is desirable because intensifying shivering is correlated with increased metabolic use of oxygen and nutrients.
Bedside assessment of shivering
A shivering score was described by Mathew et al. which ranges from 0 (no shivering) to 3 (gross muscular activity involving the entire body) and corresponds to mild fasciculation of the face, neck and Electrocardiography (ECG) disturbances in the absence of voluntary arm movement. The fundamental shivering frequency on a human electromyogram is usually in the range of 200 Hz. This is regulated by a waxing-waning clonic cycle and a sluggish tonic rhythm with 4-8 cycles per minute.
The tonic pattern, which exhibits a continuous sinusoidal form of typical shivering, is seen in intraoperative hypothermia and is thought to be thermoregulatory. However, the clonic pattern appears to be unique to recovery from volatile anaesthesia and is not thought to be a typical feature of thermoregulatory shivering. The loss of inhibition caused by general anaesthesia in the regulation of spinal reflexes may be the cause of this shivering pattern. To measure the shivering that happens in adults during TTM, the Bedside Shivering Assessment Scale (BSAS) was created. This 4-point rating system (Table 2) was verified using indirect calorimetry measurements of resting energy expenditure, oxygen consumption and carbon dioxide production [5].
| Score | Term | Description |
| 0 | None | No shivering noted on palpation of the masseter, neck or chest wall and no electrophysiological evidence of shivering (using EKG) |
| 1 | Subclinical | Electrophysiological evidence of shivering (using EKG), without clinical evidence of shivering |
| 2 | Mild | Shivering localized to the neck and/or thorax only |
| 3 | Moderate | Shivering involves gross movement of the upper extremities (in addition to neck and thorax) |
| 4 | Severe | Shivering involves gross movements of the trunk, upper and lower extremities |
Table 2: The bedside shivering assessment scale
Sixty-four percent of the fifty neuro-critical care patients who had shivering as determined by the BSAS experienced induced normothermia in order to regulate their fever. The BSAS score accurately depicted the beginning and continuing metabolic stress experienced during shivering. In contrast to patients with a BSAS score of 0 to 1, who expended roughly 1390 to 1730 kcal/d, those with a score of 2 to 3 were linked to a resting energy expenditure of 2303 to 3686 kcal/d.
These findings suggest that the BSAS is a useful method for figuring out how shivering affects metabolism. The lower brain metabolic rate is one advantage of mild hypothermia. Consequently, the advantages of TTM may be offset by untreated shivering. It is evident from the rise in metabolic consumption linked to shivering that patients with a BSAS score of 1 or higher should be treated and their shivering should be evaluated. To track the success of therapies to lessen shivering, ongoing assessment is essential.
Objective assessment of shivering via technology
Another objective method of evaluating shivering is to use electrodes to measure muscle activation. EMG recordings have been used in the past to study shivering. An EMG "waxing and waning pattern" of waves with a tremor frequency of 200 Hz or 4 to 8 cycles per minute, is produced during shivering. EMG electrodes are often positioned bilaterally on the primary muscle groups of the pectorals because this is where shivering typically begins. The EMG system is difficult to use; it needs sophisticated testing, specialised equipment and data interpretation. The ICU is not a practical place to use continuous EMG to assess shivering, unlike the lab or clinic. As a result, it appears appropriate to employ the BSAS in clinical practice to evaluate patients for shivering.
Therapeutic strategies for the treatment of shivering
Strategies to counter shivering include both nonpharmacological and pharmacological interventions. The methods chosen to counter shivering should begin with the least invasive and proceed to more aggressive means.
Non-pharmacological methods
Surface counter-warming is a noninvasive, nonpharmacological treatment for shivering. Prior clinical research 34 concentrated on locally warming patients' hands, feet and faces to lessen shivering. It is believed that at least 20% of the shivering threshold is influenced by skin temperature. The shivering reaction may be lessened by the 4°C increase in mean temperature caused by skin warming. In a recent study, 50 neuro-critical care patients who needed fever management and hypothermia had the effects of surface counter-warming of the complete body examined. These techniques function by either keeping or raising body temperature beyond the shivering threshold or by using sensory input from warmer skin to obstruct the central shivering reflex [6].
In perioperative and induced hypothermia settings, active coetaneous warming which includes electric heating, watercirculating garments, forced air and radiant heating is an efficient way to manage shivering. On the other hand, data indicates that body core warming (heated fluid, heated air) and passive coetaneous warming (cotton blanket, elastic bandage) are, at most, minimally beneficial Active coetaneous warming was linked to the highest proportion of favourable outcomes in clinical settings involving surgery or artificially induced hypothermia, according to Park B, et al.
It may be possible to reduce core temperature loss by using forced air warming in conjunction with heated IV fluids. In the event of redistribution hypothermia, warmed intravenous fluid may prevent a drop in body temperature; while forced-air warming warms the patient from the outside in warmed IV fluid is a useful technique for reducing peri-operative hypothermia, according to several investigations.
Pharmacological methods
Shivering is a thermoregulatory reaction that can be treated and blunted by a number of pharmaceutical medications. In order to successfully lessen the shiver reflex, the drugs are frequently administered in concert. Centrally acting analgesics (like tramadol), opioid receptor agonists (like meperidine, fentanyl), cholinesterase inhibitors (like physostigmine) and N-methyl-Daspartate receptor antagonists (like ketamine, magnesium sulphate) were the most effective pharmaceutical groups for ant shivering. Relatively less effective classes included α2-central agonists (clonidine, dexmedetomidine), ant serotonergic medicines (ondansetron) and anti-inflammatory medications (dexamethasone) [7].
Opioid receptor agonists
Meperidine acts primarily on the central nervous system by activating the κ and μ-opioid receptors, which is likely the reason behind its therapeutic effect on PS. Pethidine has an antishivering effect by lowering the shivering threshold and causing a reduction in core temperature, making it the only opioid that is an agonist at both the μ and κ receptors that are closely linked to the aetiology of shivering. Since meperidine's equi-analgesic dose is significantly more effective in preventing shivering than other opioids like morphine, fentanyl, alfentanil or sufentanil, it is the most often used intravenous medication for treating and preventing shivering.
Anti-serotonergic agents
Opioid and non-opioid drugs are often used to treat postoperative shivering, but they have potential side effects, including hypotension, hypertension, sedation, respiratory depression, nausea and vomiting. More recently, 5-HT3 receptor antagonists have emerged as means of preventing postoperative shivering. According to meta-analysis by Zhoy C, 5-HT3 receptor antagonists appear to prevent postoperative shivering, with a broadly comparable efficacy to meperidine.
The preoptic area of the hypothalamus releases 5-HT 3 to activate heat production pathways and thus increase body temperature. 5-HT3 antagonists may prevent postoperative shivering by inhibiting reuptake of 5-HT in the preoptic area. 5- HT3 antagonists effectively prevent postoperative shivering after general anesthesia and spinal anesthesia. According to Li M, et al., meta-analysis treatment with ondansetron is safe and may reduce PS. This finding encourages the use of ondansetron to prevent PS. Palonosetron is a new generation of a 5-HT3 antagonist which did not influence perioperative hypothermia or PAS [8].
Α2-receptor agonist
Medications targeting alpha 2 adrenergic agonist receptors have been utilised in an attempt to minimise postoperative shivering due to their potential to cause decreased sympathetic activity and central modulation of vasoconstrictor tone. Clonidine and dexmedetomidine have been shown to minimise postoperative shivering, however dexmedetomidine may cause patients to become more sedated, according to the Cochrane study. But this evidence is of very poor quality. There are many options for dosages, delivery schedules and timing: intraoperative or preoperative, oral or intravenous. When given intravenously as a preventative measure, dexmedetomidine lowers the risk of postanesthetic shivering in individuals receiving general anaesthesia.
N-methyl-D-aspartate receptor antagonist
Magnesium sulphate is a non-competitive antagonist of NMethyl-D-Aspartate (NMDA) receptors and a naturally occurring calcium antagonist. Because the medication has both a central action and a modest muscle relaxant effect, it may simultaneously lessen the development of shivering, which is the gradual increase in shivering severity with hypothermia. PS was decreased by magnesium sulphate intraoperatively. Postoperative shivering can be effectively treated with magnesium.
It only slightly lowers the shivering threshold; however, many postoperative patients have core temperatures that are only marginally below the typical shivering threshold. Magnesium may therefore be adequate to lessen postoperative shivering. Magnesium (bolus 50 mg/kg IV and 15 mg/kg/h continuous infusion) during a total intravenous anaesthesia based on propofol-remifentanil has been shown by Ryu, et al. to decrease PONV and postoperative shivering.
Ketamine is a noncompetitive antagonist of NMDA receptors that inhibits postganglionic NE uptake to produce a central sympathomimetic action. Reduced heat diffusion from the core to the periphery is one of its impacts. In a trial conducted by Lalnunmawii Sailo, et al. with 90 patients aged 18 to 60, a prospective randomised, double-blind, placebo-controlled study found that 0.5 mg/kg and 0.25 mg/kg of ketamine significantly reduced shivering in patients undergoing elective infra-umbilical surgery under subarachnoid blocks [9].
The incidence of shivering was found to be significantly lower in the ketamine group (41.5%) as compared to the saline group (70.7%; p=0.028) in Lema, GF, et al.'s study. It was also found that prophylactic administration of low-dose IV ketamine is effective for reducing the incidence and intensity of shivering and can be recommended for patients undergoing spinal anaesthesia. Likewise, research conducted by Hasannasab B, et al. and Xue X, et al. have demonstrated the efficacy of ketamine in preventing postoperative shivering.
An RCT conducted by Hasan Zabetian, et al. on thirty pregnant women indicates that ketamine 0.3 mg/kg is superior to ketamine 0.15 mg/kg in terms of managing shivering and other post-surgery adverse effects. According to studies, ketamine may prevent shivering at doses of 0.5 mg/kg or less. This dose is far lower than the amount needed to induce general anaesthesia and 0.5 mg/kg of ketamine is unlikely to cause ketamine-related adverse effects.
Ketamine-pethidine
Hasan Zabetian, et al. RCT study found that among 45 women, 0.3 mg of ketamine per kg is superior to 0.15 mg of ketamine per kg in terms of reducing postoperative shivering. Our results, however, still indicate that pethidine is the better standard drug to avoid postoperative shivering. When utilising pethidine is restricted, ketamine might be used due to its availability and analgesic qualities. Ketamine at a dose of 0.75 mg/kg was found to reduce shivering in some studies when compared to pethidine; however, it also caused side effects like nystagmus and lightheadedness [10].
According to the findings of an Indian study, ketamine doses of 0.5 mg and 0.75 mg and pethidine doses of 0.25 mg were superior for controlling shivering. The administration of a preventative low dose of injectable ketamine (1 mg/kg) was found to be beneficial in reducing post-anesthesia shivering in children undergoing tonsillectomy under general anaesthesia, according to an RCT conducted by Zabra, et al. on 120 children aged 5 to 12 years. Regarding respiratory depression, nausea and vomiting, ketamine may be theoretically superior to pethidine (0.5 mg/kg).
Ketamine-Tramadol
In reducing shivering during spinal anaesthesia in elective lower limb surgery, prophylactic intravenous ketamine (0.5 mg/kg) has comparable clinical efficacy to intravenous tramadol (0.5 mg/ kg), according to the results of an RCT conducted among 90 patients by Amir Nazir, et al. The hemodynamic parameters and side effects did not vary significantly.
Ketamine-Midazolam
The results of the RCT showed that low-dose midazolam 0.02 mg/kg with ketamine 0.25 mg/kg prevented shivering during the spinal anaesthesia required for emergency lower limb surgery with similar efficacy to that of a larger dose of midazolam 0.04 mg/kg plus ketamine 0.25 mg/kg. The hemodynamic parameters remained unchanged across all groups. On the other hand, nystagmus was more common and individuals in the midazolam plus ketamine groups were more subdued. In order to prevent shivering-related regional anaesthesia, Honarmand and Safavi's research shown that the prophylactic use of intravenous ketamine 0.25 mg/kg and intravenous midazolam 37.5 μg/kg was more successful than intravenous ketamine 0.5 mg/kg or intravenous midazolam 75 μg/kg.
Ondansetron-Ketamine
In pre-medicated patients undergoing ENT surgery under general anaesthesia, prophylactic administration of a combination of intravenous ondansetron 4 mg plus ketamine 0.25 mg/kg is comparable to either ondansetron 8 mg IV or ketamine 0.5 mg/kg IV in preventing post-anesthetic shivering, according to a prospective, randomised, double-blind, placebocontrolled study conducted by Abdelhalim, et al. among 120 patients. This was observed without causing any delays in recovery or discharge from the intensive care unit. But when ketamine at a low dose of 0.25 mg/kg was combined with ondansetron 4 mg, the likelihood of a sedative effect or hallucination was lower than when ketamine at a dose of 0.5 mg/kg was administered. Ondansetron also has benefits as an anti-shivering agent due to a lower rate of Post-Operative Nausea and Vomiting (PONV). In patients undergoing spinal anaesthesia, Shakya, et al. reported that low doses of ketamine 0.25 mg/kg and ondansetron 4 mg significantly decreased the incidence of shivering to 2.5% and 10%, respectively. The mean patient weight in the Kelsaka and Shakya studies was 52.80 kg, whereas in the Shakya study it was 76 kg.
Other drugs
There are many medications available that are intended to treat and prevent PS. Physostigmine inhibits PAS via the cholinergic system, however it can also elevate blood pressure, induce nausea and vomiting and raise heart rate. Doxapram has been shown to be beneficial on PAS when administered as a stimulant in respiratory failure; nevertheless, it has a noticeable adverse effect on hemodynamics. Under general anaesthesia, hydrocortisone (1-2 mgkg-1 I.V.) is an efficient prophylactic measure against postoperative shivering in patients having day care knee arthroscopies. One of the most researched and successful anti-shivering medications is nemopam, a centrally acting analgesic that inhibits synaptosomal reuptake of multiple neurotransmitters, including dopamine, NE and serotonin.
Future directives and areas of controversy
Since the studies on the use of ketamine included in this review were not strong enough to confidently derive strong recommendation for their effectiveness in management of PAS with appropriate dose, further studies on the dose dependent effectiveness of using ketamine for PAS management, even in resource limited areas may find the optimal dose of ketamine for this purpose. There is a controversy on the use of ketamine 0.15 mg/kg and 0.5 mg/kg for the PAS management in surgical patient regarding effectiveness and side effects respectively. There are two studies one on effectiveness and one on efficacy of ketamine 0.15 mg/kg with contradicting outcome (Figure 2).
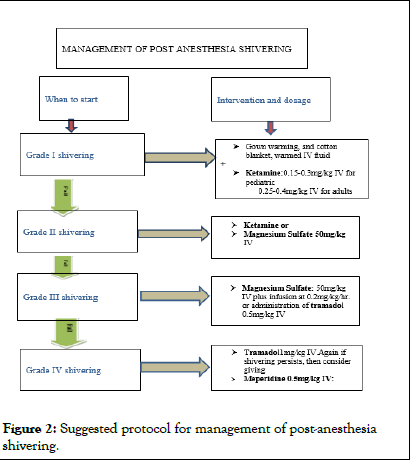
Figure 2: Suggested protocol for management of post-anesthesia shivering.
Various drugs have been used to treat or prevent post-anesthesia shivering, but the ideal treatment has not yet been found. Most studies suggest that the low dose of ketamine 0.15 mg/kg is effective in managing post-anesthetic shivering without causing any complication but, few in contrary, suggest that even though it doesn’t cause complications, it has questionable efficacy in preventing PAS after general anesthesia, while lower doses do not seem to confer any advantage at all. On the other hands, the use of ketamine at a dose of 0.5 mg/kg which performs better than placebo and possibly is even equally effective to meperidine, but reported that it may cause side effects like hallucinations and delirium. We recommend the use of ketamine to prevent or treat PAS with the doses 0.15 mg/kg to 0.5 mg/kg to get effective management of PAS and thereby to prevent its side effects at higher doses. Despite the number of studies concerning regional anesthesia, the diverse regimens used in each study makes it infeasible to draw safe conclusions. In general, the rationale for the use of ketamine in the setting of PAS is that it could serve as an alternative drug for patients with bradycardia, hypotension, respiratory depression, nausea, vomiting or allergic reactions to pethidine.
None.
WWW funded the research.
Hawasa University college of health science and department of anaesthesia informed consent obtained from parents.
None declared.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Angasa D, Eshetu O, Belay F, Zemedkun A, Muka M, Abebe M (2025) Evidence Based Clinical Guideline on the Use of Ketamine for Management of Post-Anesthesia Shivering in Surgical Patients. J Anesth Clin Res. 16:1174.
Received: 06-Jun-2024, Manuscript No. jacr-24-31906; Editor assigned: 11-Jun-2024, Pre QC No. jacr-24-31906 (PQ); Reviewed: 25-Jun-2024, QC No. jacr-24-31906; Revised: 10-Feb-2025, Manuscript No. jacr-24-31906 (R); Published: 17-Feb-2025 , DOI: 10.35248/2155-6148.25.16.1174
Copyright: © 2025 Angasa D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.