Fisheries and Aquaculture Journal
Open Access
ISSN: 2150-3508
ISSN: 2150-3508
Research Article - (2022)Volume 13, Issue 6
Fish sperm motility nowadays is considered as the best biomarker for the quality of fish spermatozoa, and sperm motion parameters from more than 300 fish species have been reported in more than 1500 scientific articles covering a wide range of topics, from molecular biology to ecology. The present study was conducted to evaluate two lab prepared activation media (A, B) and control (distilled water) on the milt quality in male and fertilization rate in female American channel catfish (Ictalurus punctatus). Brooders were developed from locally bred catfish seed and selected on the basis of maturity at Aquaculture and Fisheries Program, National Agriculture Research Center (NARC), Islamabad Pakistan. The experiment used a Complete Randomized Design (CRD) with three replicates for each of the three treatments: media A, B, and Control (distilled water). As millions of sperms are present in per micro liter of sperm therefore, for more effective use of sperm or milt, different types of activation or dilution media were used. After collection of milt it was diluted with the above corresponding activation media in ratio of 1:29 followed by in-vitro analysis for various parameters including sperm mobility, sperm mobility duration and sperm viability. Fertilization rates was checked by using same diluted milt aliquots and the results revealed that the components were significantly different (p<0.05) in the prescribed conducts. The sperm/egg aliquot treated with medium "B" had the highest mobility/motility and fertilization rates (81.66% ± 2.88 to 85.33% ± 2.08), followed by media "A" (73.33% ± 2.80 to 71.66% ± 2.08), and control (68.33% ± 2.8 to 67.33% ± 2.08). Sperm viability and concentration in A, B, and C, or the control, were likewise substantially different (p<0.05) from one another. The media “B” had the greatest values (61.66% ± 2.53 to 2.50% ± 0.14), followed by "A" (57.33% ± 2.51 to 2.37% ± 0.76), and "Control" (48.33% ± 1.15 to 1.93% ± 0.45). The length of the sperm mobility duration varied considerably (p<0.05) across all treatments. It was measured in seconds and was 320sec ± 34.64 for the control, 570sec ± 43.58 for media "B" alone, and 471sec ± 10.14 for media "A" with means and standard deviations.
Activating media, In-vitro fertilization, Sperm mobility, Sperm viability
The efficiency of reproduction depends on the quality of both gametes (eggs+sperm) [1]. This is the foremost aspect that donates to fruitful fertilization and development of viable descendants. The appropriate estimation of gamete quality is the best implement for defining the potential fertility of a sample. Using sperm from multiple males is in general in the production of many commercial species. However, fish sperm motility assessment as a tool for aquaculture research: A historical approach. Male can lead to sperm competition resulting sperm variability and, consequently, all the males cannot equally contribute to gene pool. In this intellect, the precise estimation of individual spermatozoa features, such as motility, is necessary to achieve biased info concerning separate male fecundity potential. However, there are many innovations that can provide a reliable sperm analysis, Computer-Assisted Sperm Analysis (CASA) [2].
Sperm activation is essential for reproduction because with in the short motility duration activated sperm are able to reach, impasse and enter eggs, and pledge fertilization [3]. For induce spawning motility activation is very important for sperm quality evaluation and also to develop protocols for research activities to address sperm competition. Numerous physicochemical aspects play vital roles in inducing motility activation of fish sperm. Hypotonic solutions are used for inducing motility in most of freshwater fishes, while hypertonic solutions are used for saltwater fishes [4]. For euryhaline species, such as Medaka (Oryzias latipes) and Tilapia (Oreochromis mossambicus) hypotonic, isotonic, or hypertonic media can be used for sperm motility activation [5]. Motility can be activated in some species by electrolytic and non-electrolytic solutions with in certain ranges of osmotic pressure. However, in some species, in addition to or as an alternate to osmotic pressure concentrations of ions are critical to initiate motility. For example in salmonids high levels of K+ concentration are a major inhibitor of sperm motility, preceding to producing, while Ca+ is an antagonistic to this inhibitory effect [6].
The catfishes are gaining popularity in the culture system because of their high growth, consumer preference due to good quality flesh with very few spines and high export potential. One of the high-value fish species American channel catfish (Ictalurus punctatus) was successfully introduced in aquaculture system of Pakistan. The breeding of channel catfish Ictalurus punctatus carried out for seed stock production including the pond culture, the pen and the aquarium culture methods (NASS, 2004). Sperm motility gets enhanced for longer period of time by any artificial media that impart better activation of sperm and prevent their exposure to extreme osmotic conditions [7]. As the collection of milt from channel catfish by hand stripping is impossible, therefore the males are sacrificed, testis are taken out of the body to squeeze out the milt.
Assessment and establishment of fish semen quality parameters would greatly facilitate adoption of various reproductive technologies in aquaculture. This study therefore evaluated sperm mobility, viability and concentration on fertilization dimensions in Channel catfish using two types of laboratory prepared activating solutions with dissimilar chemical configuration e.g. Potassium Chloride (KCl) including Sodium Chloride (NaCl) and Tris as compared with control i.e. distill water.
Study area
The study was conducted during the months of April-July 2021 at Aquaculture and Fisheries Program (AFP), Animal Science Institute (ASI), National Agricultural Research Centre (NARC) Islamabad under Pakistan Agricultural Research Council (PARC), Ministry of Food Security & Research Islamabad, situated in Pothohar area near Chak Shahzad NIH. The program has three well equipped research laboratories including fish microbiology, fish limnology and fish nutrition lab. Besides there are earthen research ponds, along with channel catfish and tilapia fish seed production hatcheries, and concrete raceways, hatching troughs, fiber glass circular tanks and tube wells.
Experimental design
The present study was intended to investigate two types of laboratory prepared sperm motility activation solutions or media e.g. A and B including C i.e. distilled water (control) for sperm mobility or motility and egg fertilization rates in locally bred American Channel catfish (Ictalurus punctatus). The experimental design was CRD (Complete Randomized Design) with three treatments (A, B and control) and three replicates.
Preparation of media
Activating solutions (media) were prescribed at Aquaculture & Fisheries Program (AFP) as categorized by Horvath and Bastami separately, with dissimilar chemical (MERCK) conformations accordingly and predominantly by potassium K+ ion concentration including chemical tris in 10 milliliter (ml) of distilled water [8]. Media “A” was prepared by adding 45 millimolar (mM) of NaCl equivalent to 0.0262 gm, 05 millimolar (Mm) of KCl equivalent to 0.00372 gm and 30 millimolar (mM) of Tris equivalent to 0.0363 gm in 10 milliliter (ml) of distilled water while Media “B” was prepared by adding 50 millimolar (mM) of NaCl equivalent to 0.0292 gm, 30 millimolar (mM) of KCl equivalent to 0.0224 gm and 30 millimolar (mM) of Tris equivalent to 0.0363 gm in 10 milliliter (ml) of distilled water.
Experimental procedure
Brood stock preparation and selection: American Channel catfish brooders were prepared at Aquaculture & Fisheries program, NARC farmed ponds under captive conditions feeding with 30% - 35% Crude Protein (CP) level artificial diets. Identified and selected channel Catfish brooders on the basis of maturity approaches including 06 males and 06 females with an average weight of (700-900) gm for females and (900-1000) gm for males, respectively, at the time of experimental work all the fish samples were healthy and almost disease free and fully ripened at their state during the breeding season i.e. May- August. Immediately after catching, the fish were carried to laboratory for dissections and collection of gametes for evaluation of sperm mobility and eggs fertilization.
Sperm mobility: Sperm mobility was observed under microscope using glass slides by taking 01 (µL) of milt+29 (µL) of activating media=30 (µL) diluted solution, from a selected aliquot. Then using cover slip on the slide, it was observed under the microscope with 100x magnification. Sperm mobility was measured as the proportion of increasingly moveable spermatozoa in each stimulated field. Only aggressively stirring spermatozoa were measured as motile and other cells that tremble in the same field were considered immotile.Mobility duration: Sperm mobility time calculated as the total time consumed by all the spermatozoa cells converted from completely mobilized till to 100% of spermatozoa’s become dead. For the observation of motility duration a drop of 30 µL sample of diluted sperm (01 µL milt+29 µL activating solution=30 µL) dilution was reserved from selected aliquot on glass slide, then using cover slip observed under 100x magnification. Both the parameters i.e. motility and its time period both were recorded after regular interval of a second in lab conditions. After every one to two seconds interval samples were checked and perceived the percentage of mobilized spermatozoa until all were found dead.
Sperm viability: For sperm viability evaluation used the stain process, Trypan blue (0.4 percent) solution was prepared dissolved with four milli gram (mg) of trypan in one milliliter (ml) of distilled water. Before usage, the stain was filtered all the time. Smear was prepared on the glass slide adding with 01 µL of sperm and 01 µL of stain taken and after mixing well, it was permitted to air dry for 2-5 minutes and observed at 40x magnification under microscope. When milt was mixed with stain live spermatozoa with whole membranes remained unstained, whereas sperms that were dead and their membranes were not intact stained blue. Sperm viability was further calculated by using following formula:
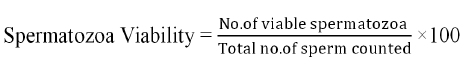
Concentration/microliter: Concentration of spermatozoa was calculated by using Neuberger hemocytometer as mentioned in standard method. A total drop of 30 µL diluted solution (01 µL of milt+29 µL of media) was loaded on the hemocytometer with the help of micropipette. While loading, the tip of pipette was placed on V-shaped groove of hemocytometer. The sample was placed at lab conditions to settle the movement of spermatozoa. The concentration was assessed by counting the spermatozoa in four corner squares and in Central Square using 40x magnifications. Sperm concentration of per microliter was determined by following formula:
Viable sperm cells per square × dilution factor × 104
Eggs collection and fertilization rates: Eggs collected from selected females and divided into three batches 01 gm consisting of almost 220 eggs and then sperm collected from males and separated into three aliquots, as per determined sperm concentration/microliter. Then 30 µL of ready solution was added entirely. Dissimilar volumes of milt were used to maintain the spermatozoa concentration per microliter in almost all the treatments.
In the current study fertilization rates were evaluated visually as the percent of eyed eggs with respect to the total number of eggs after fertilization. This diluted milt was mixed with eggs. After mixing for 5 minutes, fresh water was added and allowed to stand for 10-15 minutes for better fertilization. Water hardened and fertilized eggs were bright looks like a gems with three to five millimeter in dia and also translucent in color, dead or unfertilized eggs seems impervious. Fertilization rates were determined using the following formulae.
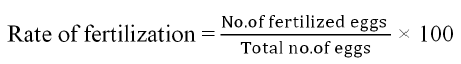
Statistical analysis: Obtained results were mentioned as means ± Standard Deviations (SD). Effects of media was measured by One-way ANOVA (variances of analysis) and also used (DMRT) Duncan’s Multiple Range Test using software SPSS for significant p<0.05 comparison of average means.
Inspected the effects of two lab-prepared sperm mobility Activation Media influence the quality factors i.e. sperm mobility within a time period and viability & fertilization rates as compared with distilled water i.e. control, in American Channel Catfish (Ictalurus punctatus).
Sperm mobility
Results obtained in the current study revealed that within the experimental activation media within two treatments and compared with control i.e. distilled water the highest values found significant p<0.05 effects with in B i.e. 81.66% ± 2.88 followed by A i.e. 73.33% ± 2.80 and distilled water (control) was 68.33% ± 2.88. Data also presented in Figure 1 with means± Standard Deviation (S.D).
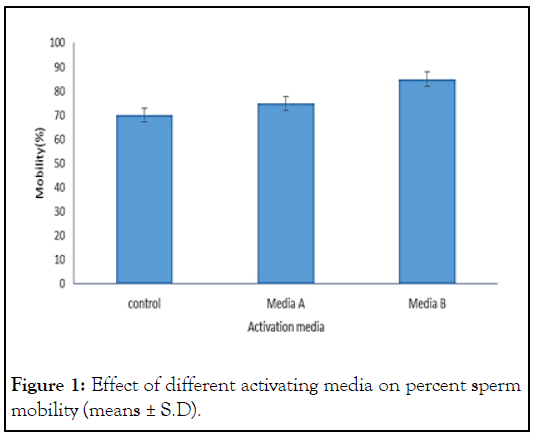
Figure 1: Effect of different activating media on percent sperm mobility (means ± S.D).
Sperm mobility duration
Calculated in seconds, duration recorded with A. 471sec ± 10.40, B. 570sec ± 43.59 and control, 320sec ± 34.65 seconds, significantly p<0.05 dissimilar from each other. It is shown in Figure 2 with means ± Standard Deviation (S.D).
Figure 2: Effect of different activating media on sperm mobility duration (means ± S.D).
Sperm viability
Media A. 57.34% ± 1.52, B. 61.67% ± 2.53 and C. 48.34% ± ![]() 1.52, highest B i.e. 61.67%, followed by others mentioned with average with means ± Standard Deviation (S.D) in Figure 3. Significantly p<0.05 indicates that within the media A, B and C (control) viability differs significantly.
1.52, highest B i.e. 61.67%, followed by others mentioned with average with means ± Standard Deviation (S.D) in Figure 3. Significantly p<0.05 indicates that within the media A, B and C (control) viability differs significantly.
Figure 3: Effect of different activating media on percent sperm viability (means ± S.D).
Sperm concentration/micro liter
Sperm concentration was determined to adjust the almost equal concentration of sperms in each aliquot of dilution media for determining the fertilization rate. The value of concentration was 2.37 × 104 ± 0.76, 2.50 × 104 ± 0.14 and 1.93 × 104 ± 0.45, for media A, B and control.
Fertilization rates
Fertilization rates under the almost equalized concentration of spermatozoa obtained were 71.66% ± 2.88, 85.33% ± 2.08 and 67.33% ± 2.08 for media A, B & distilled water (control) separately. The highest percentage of fertilization rates were observed in activation media B i.e. 85.33% followed by A and C (control). Data observed by statistical analysis that the fertilization rates in all the treatments were found varied significantly p<0.05 from each other. Data also presented with means ± Standard Deviations (S.D) in Figure 4.
Figure 4: Effect of different activating media on percent fertilization rate (means ± S.D).
The results obtained in the present study indicate that the type of Activating Solutions (AS) used has a significant effect on milt mobility and fertilization rate in American Channel catfish. Fertilization success is firmly related to the quality of gametes, the activating solution used, the sperm to egg ratio, the time of contact active sperm with active eggs [1,4]. Many studies carried out regarding investigation of different activation media on the motility activation of spermatozoa [7]. However, there is a very little knowledge on the effect of different activating solutions on fertilization effectiveness [4].
The spermatozoa of most fresh water fish species are immotile in the presence of semen plasma or isotonic solutions, and to obtain good motility, they must be diluted with suitable medium. The objective of current study was to identify the best solutions for Channel catfish sperm and motilities were compared using laboratory prepared activating solutions/media to in vitro fertilization [9-10]. The effect of sodium and potassium and as well as optimal pH on the motility activation of common carp L. sperm during short term storage in artificial seminal plasma was investigated by researchers, immediately after dilution (0 h), sperm motility was high range 81.5%-93.6% in all ASP variants and even 98.4% in control group with fresh sperm while with the passage of time motility significantly dropped to 12% [11-14]. In the current study motility observed 81.66% with fresh sperm using activation media by adding 50 millimolar (mM) of NaCl equivalent to 0.0292 gm, 30 millimolar (mM) of KCl equivalent to 0.0224 gm and 30 millimolar (mM) of Tris equivalent to 0.0363 gm in 10 milliliter (ml) of distilled water [14-16].
In the current study highest sperm motility duration was achieved e.g. 570 sec. similarly using the same type of activation media, duration was achieved up to between 10 to 15 minutes in Cyprinus carpio and Danio rerio. Alternative results were also obtained i.e. 540s using sodium citrate and others. The sperm viability analysis, the animate and deceased proportion of spermatozoa was intended by stain process of trypan blue (0.4 percent) milt trial and the libel was detected fewer than 40x magnification in microscope. Comparable studies stayed completed and suggested the stain of sperm by calculating the number of whole (viable and damaged red-stained) spermatozoa under microscope. The potassium ion amplified sperm viability and rapidity of sperm ambition at an absorption underneath in the seminal plasma, where Na ion and electrolytes were minus operational. The researchers guided that species specific, and greater eggs also take a developed sperm viability comparatively in small eggs. However, catfish produces larger eggs.
In the current study fertilization rates were noted in American Channel catfish is around 85.33% with mean standard deviations 2.88% in media (B), related consequences regarding substantial high fertilization rates i.e. 86.7%. It can be generally high with special treatments. The object may be recognized to the enormous alterations of hormone dosages, and also depending on the size of brooder including seasonal dissimilarity. Fertilization rates are firmly connected to the superiority of gonads, and egg to sperm proportion, the period of interaction of energetic sperm with vigorous eggs and the activating solution used. The optimal ratio of spermatozoa:eggs (1500:1) for artificial insemination of African catfish Clarias gave fertilization and hatching rates of 80 and 68% respectively.
Citation: Ahmed A, Kauser R, Basharat H, Ali R, Soomro AN (2022) Evaluation of Sperm Mobility Activation Media for Egg Fertilization in Locally Bred American Channel Catfish (Ictalurus Punctatus). Fish Aqua J.13: 313.
Received: 26-Oct-2022, Manuscript No. FAJ-22-19829; Editor assigned: 28-Oct-2022, Pre QC No. FAJ-22-19829 (PQ); Reviewed: 11-Nov-2022, QC No. FAJ-22-19829; Revised: 18-Nov-2022, Manuscript No. FAJ-22-19829 (R); Published: 25-Nov-2023 , DOI: 10.35248/2150-3508.22.13.313
Copyright: © 2022 Ahmed A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.