
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2023)Volume 13, Issue 4
Skin sensitization is classified as a type IV (delayed) allergy (allergic contact dermatitis) caused by T lymphocyte activation that manifests as an inflammatory reaction within 48 hours of contact with a causative allergen. It can directly decrease the quality of life for those who are afflicted. Therefore, to appropriately determine the potential of chemical substances to sensitize skin is extremely important.
The development of alternatives to animal tests according to the Adverse Outcome Pathways (AOPs) of skin sensitization has recently progressed and test guidelines have been established by the Organization for Economic Cooperation and Development (OECD). The potential of chemicals to sensitize skin is difficult to determine using a single test and an alternative method that precisely reflects the results of animal experiments is not available. The coverage of alternative methods also needs to be precisely determined. Under these circumstances, the OECD issued Defined Approaches (DAs) for skin sensitization, which precisely evaluates skin sensitization by combining in vitro, in chemico and in silico methods. Based on this consideration, various evaluation methods have been combined, and issues such as their coverage have been clarified.
This review outlines the development of alternative skin sensitization tests and global trends in evaluations using such tests. We also review the Amino Acid Derivative Reactivity Assay-Organic Solvent (ADRA-OS), which we developed as an alternative to evaluating the skin sensitization potential of chemicals. The results of epoxy compound evaluations and the influence of chemical structures are considered.
Skin sensitization; Alternative method; Amino acid derivative reactivity assay-organic solvent; Epoxide
Allergic Contact Dermatitis (ACD) is an inflammatory reaction of the skin triggered by contact with external sensitizers. It develops as a result of type IV hypersensitivity caused by the activation of allergen-specific T lymphocytes [1]. Chemical substances can cause skin sensitization by permeating the skin and then binding to proteins and peptides. This promotes the formation of antigen complexes (haptens) that present on Major Histocompatibility Complexes (MHCs) in antigen-presenting cells. Costimulatory signal(s) can be sufficient to activate and expand T-cells, which results in an allergic response [2]. Allergic contact dermatitis can directly reduce the quality of life for those afflicted. Appropriate determination of the potential of chemical substances to sensitize skin is extremely important for both industrial and consumer products.
Skin sensitization has conventionally been assessed using the Guinea Pig Maximization Test (GPMT), the Buehler Test, and the mouse Local Lymph Node Assay (LLNA). The LLNA is a regulatory standard for evaluating the skin-sensitizing potential of chemicals [3]. Regulatory authorities still occasionally request data from these tests because they are deemed important for judging the skin sensitization potential of chemicals. However, the 7th Amendment of the European Council Cosmetics Directive (76/768/EEC) [4], introduced new regulations to the Europe (EU) in March 2003, regarding cosmetics and evaluations of cosmetic raw materials without using animals. The EU then banned the sale of cosmetics and their ingredients tested on animals in March 2009, regardless of the product origin. The European Registration, Evaluation, Authorization and Restriction of Chemicals regulation (EU REACH legislation) requires the use of methods without animals, but mandates animal tests as a last resort [5]. However, the 2016 update requires in vitro and in silico methods as the first choice for assessing skin sensitization [6]. This means that animal tests are permitted under exceptional circumstances [5]. Under these circumstances, accurate evaluation of skin sensitization potential using alternatives to animal tests (in vitro, in chemico) and in silico are desirable.
Application of Defined Approaches to evaluating skin sensitization
Animal test alternatives for assessing skin sensitization have been developed and Organization for Economic Cooperation and Development (OECD) test guidelines have been established according to the four Key Events (KEs) that comprise Adverse Outcome Pathways (AOPs). These events comprise covalent binding to Epidermal Proteins (KE 1), Keratinocyte Activation (KE 2), Dendritic Cell Activation (KE 3), and Lymphocyte Proliferation/differentiation (KE 4). Alternative tests for KE 1 comprise chemico Direct Peptide Reactivity Assay (DPRA) and Amino Acid Derivative Reactivity Assay (ADRA) [7]. Those for KE 2 are in vitro, such as KeratinoSensTM [8], which determines the activation of the Nrf2-Keap1-ARE pathway in cells transfected with a reporter gene (Givaudan, Vernier, Switzerland). A Cell Line Activation Test (h-CLAT) uses human THP-1 cells that behave like dendritic cells exposed to sensitizers for KE 3 [9]. Thus, all alternative skin sensitization tests developed to date can evaluate only some KEs in complex allergic reactions. Presently, no single test yet has the power to predict skin sensitization potential.
The OECD has provided guidance on Defined Approaches (DAs) based on a combination of AOPs (Integrated Approach to Testing and Assessment; IATA) [10]. The OECD Guideline No. 497 includes three DAs for Skin Sensitization (DASS): 2 out of 3 DAs, Integrated Test Strategy (ITS) v1 DA, and ITSv2 DA. The DAs described in the guidelines include DPRA, KeratinoSensTM, and h-CLAT, which are alternatives to KE 1-3, and information obtained from tools in silico (ITSv1 DA: Derek Nexus, ITS v2 DA: OECD Quantitative Structure Activity Relationship (QSAR) toolbox).
However, despite the formulation of this evaluation strategy, how to operate and verify DASS prediction accuracy, evaluate formulations and mixtures, and derive quantitative information for use in risk assessment remain outstanding issues. Several case studies have addressed these issues. Twenty-seven agrochemical formulations have been evaluated using DPRA, KeratinoSensTM, and h-CLAT based on DASS. Compared with animal test data, the balanced accuracy of skin sensitization hazard prediction was 56%-78%, and the results of the “2 out of 3” evaluation were the most accurate [11]. On the other hand, the predicted balanced accuracy of ITS, which is a combination of hazard assessments by DPRA, h-CLAT, and QSAR toolbox, was 56%-57%.
The International Cooperation on Cosmetics Regulation (ICCR) announced principles for the Next Generation Risk Assessment (NGRA) of cosmetic ingredients using data and information from New Approach Methodologies (NAMs) [12]. A risk assessment of the common base ingredient in shampoos and deodorants, Diethanolamine (DEA), using NAMs such as predictions in silico as well as data from tests in chemico and in vitro has been conducted as a case study to verify a consumer risk assessment scenario (rinse-off/leave-on) combining seven DAs [13]. The risk assessment results indicated that rinse-off exposure was safe in all applied DAs. However, risk assessments for leaveon exposure led to the conclusion that not all DA combinations are safe. This indicated that there was uncertainty in the DA setting.
These findings showed that assessing skin sensitization hazards using DA is certainly useful. However, these findings indicated that far more information is needed to appropriately evaluate target chemicals and consider generated data.
Evaluation of skin sensitization by epoxy
compounds using ADRA-OS
The problem with the alternative methods, KE 1, 2, and 3, is that highly hydrophobic substances cannot be correctly evaluated [14,15]. The Epidermal Sensitization Assay (EpiSensA) was developed to expand the application coverage of alternative methods for highly hydrophobic substances for KE 2 [16]. This method was expected to correctly evaluate highly hydrophobic substances because cells are directly exposed to test substances in the same way as actual skin. However, the KE 1 method has not yet made such progress. Therefore, we developed the ADRAOrganic Solvent (ADRA-OS), which enabled the prediction of skin sensitization potential of highly hydrophobic substances (octanol/water partition coefficient (log Kow) values>6) with better precision by increasing the organic solvent ratio to 80% methanol in the reaction solution of ADRA. The ADRA-OS is an alternative KE 1 method for predicting the skin sensitization potential of test substances (1 mM) from their reactivity against N-(2-(1-Naphthyl)Acetyl)-ι-Cysteine (NAC), a nucleophile of the cysteine derivative used in ADRA. We showed that this method could evaluate highly hydrophobic substances [17].
Although ADRA-OS increases the organic solvent ratio of the reaction solution, it can dissolve water-soluble substances; therefore, hydrophilic substances with low log Kow at low concentrations can be evaluated. We evaluated the skin sensitization potential of various chemicals using ADRA-OS. Among them, we investigated the reactivity of epoxy compounds, against nucleophilic NAC from a chemical structural viewpoint.
Epoxy compounds are characterized by a highly reactive epoxy group, and are used industrially as synthetic intermediates for resins due to their wide variety and structural characteristics. However, skin disorders such as occupational contact allergic diseases and health disorders such as mutagenicity can be problematic from the viewpoint of safety because of their high reactivity. We compared the skin sensitization potential of six epoxy compounds with established animal test data (LLNA, GPMT) using ADRA-OS, and verified the animal test data (Figure 1).
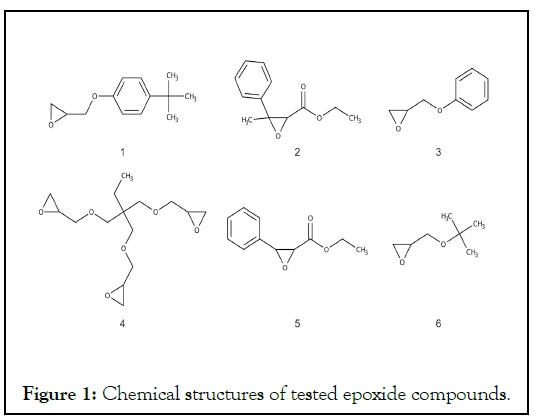
Figure 1: Chemical structures of tested epoxide compounds.
The results of epoxy compounds 1-3 were positive and consistent with the animal test data, whereas epoxy compounds 4-6 were determined as false negative (Table 1). Evaluation by ADRA-OS is based on the nucleophilic NAC depletion ratio in the reaction solution. The ADRA-OS reaction solution contains a high proportion of thiolate anions (R-S-) that function as nucleophiles. Therefore, basic triethylamine is added to the reaction solution to increase reactivity against a sensitizing substance [17]. Nevertheless, we considered that the low reactivity of epoxy compounds 4-6 with NAC might be associated with their chemical structure. Reactivity differs between cysteine residues in model peptides of skin proteins and epoxy compounds with similar chemical structures [18]. Furthermore, a comparison of the sensitization strength of epoxy compounds with structures similar to the powerful skin sensitizer phenyl glycidyl ether, using LLNA tests showed that increasing the distance between ether oxygen and epoxide moieties decreases sensitization [19]. The results of evaluations using KeratinoSensTM were similar [20]. The findings of reacting a nucleophilic model skin protein peptide with a cysteine residue against epoxy compounds showed that compounds such as phenyl glycidyl ether were very reactive against nucleophiles [21]. This was due to the stabilization mechanism of the transition state by hydrogen bonding mediated by water molecules. The hydrogen bond is thought to render the conformation of the epoxy compound more reactive with the thiolate anion of nucleophiles, thus lowering the activation energy required for the reaction.
| Epoxide (1 mM) | CAS # | MW | Log Kow | in vivo | ADRA-OS | ||
|---|---|---|---|---|---|---|---|
| NAC dep. (%) | Prediction | ||||||
| 1 | 4-tert-butylphenyl glycidyl ether | 3101-60-8 | 206.29 | -3.39 | S* | 15.9 ± 5.4 | S |
| 2 | Ethyl 3-methyl-3-phenylglycidate | 77-83-8 | 206.24 | 3.00 | S* | 6.6 ± 2.9 | S |
| 3 | Glycidyl phenyl ether | 122-60-1 | 150.18 | 1.61 | S* | 100.0 ± 0.0 | S |
| 4 | Trimethylolpropane triglycidyl ether | 3454-29-3 | 226.70 | -0.50 | S† | 5.2 ± 2.3 | NS |
| 5 | Ethyl 3-phenylglycidate | 121-39-1 | 192.21 | 2.55 | S* | 0.2 ± 0.9 | NS |
| 6 | Tert-Butyl glycidyl ether | 2426-08-06 | 130.19 | 1.08 | S‡ | 1.4 ± 1.6 | NS |
Table 1: Skin sensitization prediction of epoxides using ADRA-OS. LogKow values were calculated using KOWWIN program v.1.68 of Estimation Programs Interface (EPI) Suite™ (Environmental Protection Agency, Washington, DC, USA). In terms of ADRA-OS prediction, ≥ 5.6% and <5.6% NAC depletion were respectively considered as sensitizers, and non-sensitizers. All test chemicals were evaluated at a concentration of 1 mM. Prediction data in vivo were obtained from *ECHA, †QSAR toolbox and ‡Reference [18]. ECHA: European Chemicals Agency; QSAR: Quantitative Structure Activity Relationship, S: Sensitizers; NS: Non-Sensitizer.
Considering the results of this evaluation from a structural chemical perspective, compounds 1, 2, and 3 with an aromatic ring structure were correctly determined as skin sensitizers. However, compound 5, which has an aromatic ring structure, tested as a false negative, as did compounds 4 and 6 that do not have this structure (Table 1). Compounds 1 with a phenyl glycidyl ether structure and 3 was phenyl glycidyl ether and both tested positive. In contrast, compounds 2 and 5 with similar structures respectively tested positive and false negative. The reason for this difference in reactivity remains unknown. However, the mechanism of the transition state stabilization described above did not work in the thiolate anion reaction for compound 5.
Alternative tests established specifically for key events in an AOP are insufficient to evaluate skin sensitization, which is expressed through a complex immune response in vivo. Comprehensive evaluations of several alternative methods based on different principles and tests in silico that predict skin sensitization from the structure of chemical substances are needed. Therefore, the properties of chemical substances being tested should be understood to ensure the selection of appropriate evaluation methods and the results should be scientifically verified.
The applicability of alternative methods to individual chemical substances should be determined, case studies should be conducted, and the results generated by various test methods should be analyzed when assessing skin sensitization potential.
The authors declare no conflicts of interest.
Citation: Koizumi R, Yamaga H, Hayashi K, Watanabe S, Kataoka S (2023) Evaluation of Skin Sensitization Using Amino Acid Derivative Reactivity Assay-Organic Solvent (ADRA-OS), an Alternative to Animal Tests; Effects of Epoxy Compound. J Clin Toxicol. 13:541.
Received: 16-Aug-2023, Manuscript No. JCT-23-26105; Editor assigned: 18-Aug-2023, Pre QC No. JCT-23-26105 (PQ); Reviewed: 01-Sep-2023 Revised: 08-Sep-2023, Manuscript No. JCT-23-26105 (R); Published: 15-Sep-2023 , DOI: 10.35248/2161-0495.23.13.541
Copyright: © 2023 Koizumi R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.