Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2019)Volume 10, Issue 2
Objectives: Stability is an essential aspect in the surgical treatment of vitiligo. One or more year stability has been suggested for better surgery outcome. Recently, the chemokine CXCL10 has emerged as a putative serum marker that correlated with disease activity. The aim of the current work was to evaluate the serum level of CXCL10 versus minigrafting in predicting surgical stability in vitiligo.
Methods: Fifteen patients with 6-11 months stable disease (group 1) and fifteen patients with at least 12 months stability (group 2) were subjected to a minigraft test and evaluation of serum level of CXCL10. Repigmentation was evaluated at 3 months.
Results: Serum level of CXCL10 was not different between both groups (p >0.05). Minigraft test was positive in 40.0% in group 1 vs. 86.7% in group 2 (p=0.008). The extent of repigmentation at 3 months in patients with longer stability was significantly higher than those with shorter disease stability (p=0.023). In group 1 but not group 2, a negative minigraft test was associated with higher CXCL10 level (p=0.008). Moreover, a negative correlation was found between serum CXCL10 level and extent of repigmentation in group 1 (R=-0.670, p=0.006), but not in group 2 (R=-0.030, p=0.916). ROC analysis showed that a CXCL10 level less than 52 ng/L is associated with a positive MGT. The test had 47.4% sensitivity and 100% specificity.
Conclusion: Herein we have shown that ≥ 1 year stability is essential before attempting surgery. Minigraft test remains to be a reliable tool to detect disease stability. CXCL10 level may be helpful in patients with shorter disease stability.
Stability; Vitiligo; Minigraft; Serum; CXCL10; Repigmentation; Chemokine
Vitiligo is a common, depigmenting skin disease characterized by loss of functional melanocytes. The autoimmune theory is the leading theory in its etiology [1].
Interferon gamma (IFN- γ) is involved in the regulation of nearly all phases of immune and inflammatory responses in non-segmental vitiligo, including the activation, growth and differentiation of T-cells, B-cells, macrophages, NK cells and other cell types such as endothelial cells and fibroblasts [2-4].
Interferon gamma acts through chemokines that are small glycoproteins active for a wide variety of cell types. The IFN-γ- inducible protein (IP)-10, also called chemokine (C-X-C motif) ligand (CXCL) 10, was identified as a chemokine that is induced by IFN-γ in various cell types, such as neutrophils, lymphocytes, endothelial cells, fibroblasts, thyrocytes, keratinocytes and other epithelial cells [5-8].
CXCL10 binds to its specific receptor, chemokine (C-X-C motif) receptor (CXCR) 3 and regulates immune responses by recruitment and activation of T cells, monocytes and natural killer cells. CXCR3 is expressed not only by immune cells but also by endothelial cells, mesangial cells, thyrocytes and other epithelial cells [8,9].
Rashighi et al. [10] and Antonelli et al. [11] reported elevation of serum CXCL10 level and the expression of CXCR3 (the common receptor for CXCL9, CXCL10 and CXCL11) on melanocyte-specific CD8+ T cells in vitiligo patients. CXCL10 has been proposed as one of the serum markers for vitiligo activity [12]. However, its predictive role for surgical stability has not yet been evaluated.
Non-segmental vitiligo is either treated by medical therapies or surgery [13,14]. Stability of the disease process in vitiligo is the most important parameter to achieve a successful outcome in surgical treatment [15,16]. Evaluating stability in vitiligo depends mainly on the history obtained from the patient, which is not always reliable [17]. More objective methods in evaluating stability include, obtaining a biopsy from the margins of vitiligo patches that show absence of CD8 positive infiltrate close to melanocytes [18] or performing a minigraft test, which is evaluated 3 months later [19].
To evaluate the serum level of CXCL10 vs. minigrafting in predicting surgical stability in vitiligo.
In this prospective controlled study, 30 patients with stable vitiligo and 20 healthy completed the study. The patients were divided into two groups using the vitiligo disease activity score VIDA score [20] as follows: Group 1 (VIDA 1): 15 patients with disease stability for at least 6 months (more than 6 and less than 12 months), group 2 (VIDA 0 and -1): 15 patients with stable disease for more than a year and group 3, the healthy controls. The patients were recruited from the vitiligo outpatient clinic of the dermatology department in Ain Shams University during the period from January 2016 to January 2017. An informed written consent was taken before inclusion of the subjects into the study according to Ain Shams University Ethical Committee of Scientific Research. Inclusion criteria were: patients with stable vitiligo for more than 6 months. parameters for establishing stability of vitiligo (absence of new lesions, no extension of old lesions, absence of Koebner phenomenon based on history and examination, absence of depigmentation of repigmented lesions) [17,21]. While the exclusion criteria were: patients with active vitiligo (VIDA 2, 3, 4), patients using any topical treatment for vitiligo in the previous month or systemic treatment in the previous 3 months, vitiligo affecting > 50% body surface area, any concurrent auto-immune diseases, hemorrhagic abnormalities or anticoagulant medication, pregnant females, allergy to lidocaine, history of hypertrophic scars or keloids and any concomitant skin or other systemic disease.
Each patient was subjected to a full history taking, complete clinical examination, digital photography at a constant distance (20 cm from the lesion) under identical camera settings and lighting conditions and dermoscopic examination (Figures 1 and 2) at the time of enrollment for surgery (week 0) and at weeks 6 and 12 of follow up. Minigraft test using small biopsy punches (2 mm) was performed for every patient according to Falabella et al. [19]. The test was considered positive if unequivocal re-pigmentation takes place beyond 1 mm from the border of the implanted grafts measured by dermoscopy at week 12 (Figures 1d and 2d). On the other hand, if less than 1 mm pigment spread or no re-pigmentation was observed by dermoscopy, the test was considered negative.
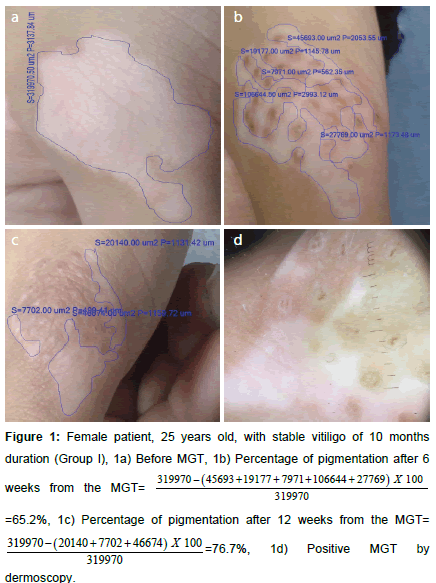
Figure 1. Female patient, 25 years old, with stable vitiligo of 10 months duration (Group I), 1a) Before MGT, 1b) Percentage of pigmentation after 6 weeks from the 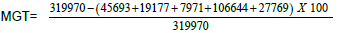 =65.2%, 1c) Percentage of pigmentation after 12 weeks from the MGT=
=65.2%, 1c) Percentage of pigmentation after 12 weeks from the MGT= 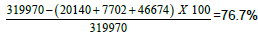 , 1d) Positive MGT by dermoscopy.
, 1d) Positive MGT by dermoscopy.
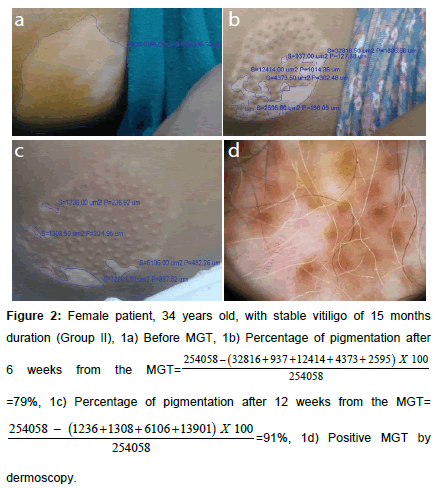
Figure 2. Female patient, 34 years old, with stable vitiligo of 15 months duration (Group II), 1a) Before MGT, 1b) Percentage of pigmentation after 6 weeks from the 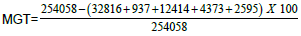 =79%, 1c) Percentage of pigmentation after 12 weeks from the MGT=
=79%, 1c) Percentage of pigmentation after 12 weeks from the MGT= 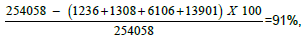 1d) Positive MGT by dermoscopy.
1d) Positive MGT by dermoscopy.
Percentage of repigmentation was evaluated by comparing the surface area of the lesion measured in μm² before (Figures 1a and 2a) and at week 6 (Figures 1b and 2b) and 12 (Figures 1c and 2c) from the MGT using image analysis software (Infinity analyze, release 6.4). A blood sample was taken for assessment of CXCL10 level in addition to evaluation of complete blood picture, prothrombin time (PT), partial thromboplastin time (PTT), pregnancy test, assessment of serum thyroid stimulating hormone (TSH), anti-thyroid peroxidase antibodies (TPO) to rule out thyroid dysfunction. Measurement of serum CXCL10 by enzyme linked immunosorbent assay technique (ELISA) was performed for patients (before minigraft test) and controls using the Human CXC-chemokine ligand 10 ELISA kit (bt-laboratory, Inc., China, Cat. No E3800Hu). The standard curve ranged from 2 ng/l- 600 ng/l. The sensitivity was 1.22 ng/l.
Group 1 (VIDA 1) included 4 males (26.7%) and 11 females (73.3%), age range 8-62 years (Mean=29.93 ± 18.98), group 2 (VIDA 0 and -1); 2 males (13.3%) and 13 females (86.7%), age range 12-50 years (Mean=22.2 ± 11.58) and group 3 included; 6 males (30%) and 14 females (70%), age range 16-53 years (Mean=26.21 ± 15.80). The three groups were matched as regards sex or age (p>0.05).
Disease duration was significantly longer in group 2 (9.93 ± 5.32 years) compared to group 1 (4.6 ± 3.26 years), (p<0.005) (Table 1). MGT was positive in a significantly higher number of patients in group 2 (86.7%) compared to group 1 (40%) (p <0.008). Similarly, the mean percentages of pigmentation at weeks 6 and 12 were significantly higher in group 2 (36.67% ± 13.45 week 6; 67.33% ± 27.31 week 12), compared to group 1 (26.67% ± 21.02 week 6; 32.33% ± 34.53 week 12) (p <0.014 week 6 and p <0.023 week 12 respectively).
| Group I | Group II | Group III | p value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (ng/L) | ± SD | Median (IQR) | Mean (ng/L) | ± SD | Median (IQR) | Mean (ng/L) | ± SD | Median (IQR) | |||
| CXCL10 | 104.47 | 92.41 | 82 (50-130) | 94.93 | 80.42 | 80 (25-140) | 40.13 | 18.62 | 40 (28-51) | 0.005* | HS |
| 104.47 | 92.41 | 82 (50-130) | 94.93 | 80.42 | 80 (25-140) | - | 1.0** | NS | |||
| 104.47 | 92.41 | 82 (50-130) | - | 40.13 | 18.62 | 40 (28-51) | 0.013** | S | |||
| - | 94.93 | 80.42 | 80 (25-140) | 40.13 | 18.62 | 40 (28-51) | 0.032** | S | |||
Table 1: Comparison between groups 1, 2 and 3 as regards the serum CXCL10 level.
Serum CXCL10 level was significantly higher in vitiligo groups compared to healthy controls (P<0.05), but not between both vitiligo groups (p=1) (Table 1).
CXCL 10 level significantly correlated between MGT positivity (p <0.006) and extent of pigmentation (p=0.006) in group 1, this was not seen in group 2 (p=0.610) and p=0.916 respectively (Figures 3a-3d).
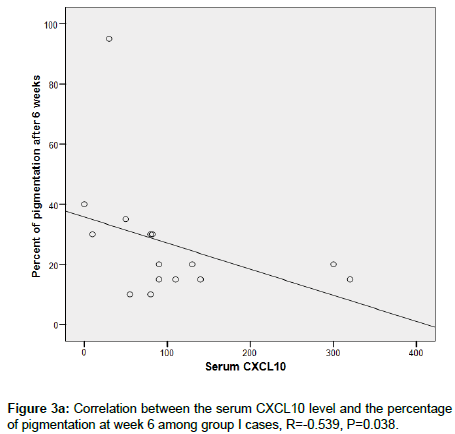
Figure 3a. Correlation between the serum CXCL10 level and the percentage of pigmentation at week 6 among group I cases, R=-0.539, P=0.038.
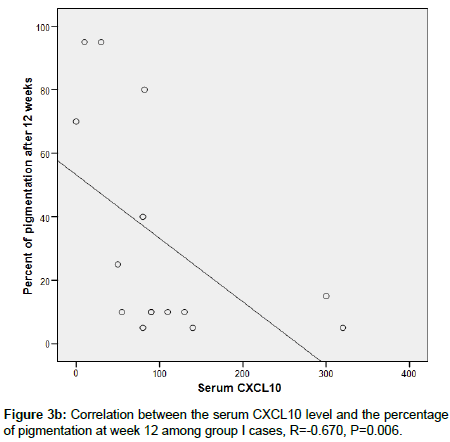
Figure 3b. Correlation between the serum CXCL10 level and the percentage of pigmentation at week 12 among group I cases, R=-0.670, P=0.006.
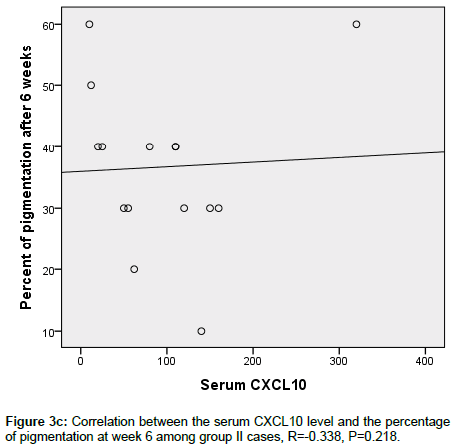
Figure 3c. Correlation between the serum CXCL10 level and the percentage of pigmentation at week 6 among group II cases, R=-0.338, P=0.218.
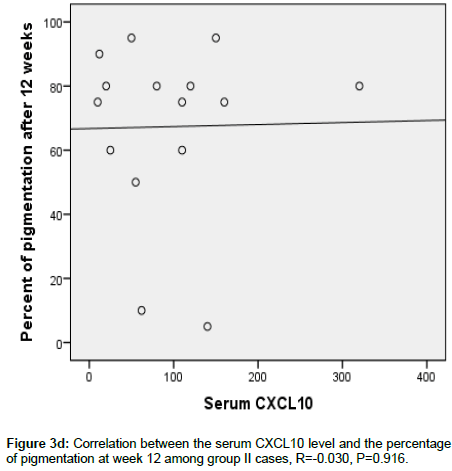
Figure 3d. Correlation between the serum CXCL10 level and the percentage of pigmentation at week 12 among group II cases, R=-0.030, P=0.916.
In order to determine a cut-off value for the specificity and sensitivity of CXCL10 in relation to MGT outcome, receiver operating characteristic (ROC) curve was done. CXCL10 level less than 52 ng/L is associated with a positive MGT. The test had 47.4% sensitivity and 100% specificity (Figure 3e).
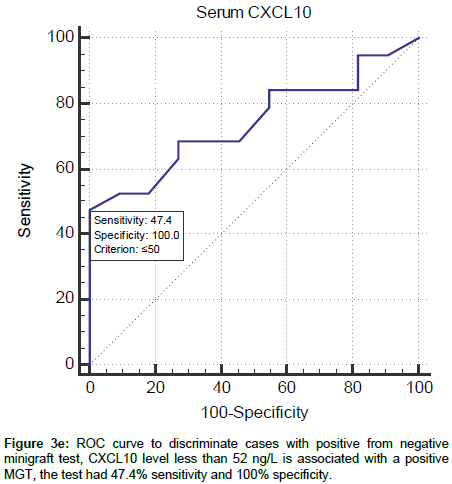
Figure 3e. ROC curve to discriminate cases with positive from negative minigraft test, CXCL10 level less than 52 ng/L is associated with a positive MGT, the test had 47.4% sensitivity and 100% specificity.
The aim of the current study was to evaluate and compare the use of CXCL10, as a serum activity biomarker, versus the well-established MGT outcome in predicting disease stability for a better surgical outcome.
Group 1 patients with VIDA 1 had a significantly lower MGT positive outcome compared to group 2 with VIDA 0 and -1. The percentage coverage of pigment was also significantly lower in group 1 compared to group 2. These data objectively emphasize the importance of the suggested stability period of more than a year for a better surgical outcome [16]. In search of previous literature, we could not find a controlled study evaluating the surgical outcome in different stability durations.
A statistically significant elevation of the serum CXCL10 level was found in vitiligo patients compared with the healthy controls, highlighting the role of CXCL10 in the immunopathogenesis of vitiligo [22,23,10]. Interestingly, we did not find a statistically significant difference in the serum CXCL10 level in group 1 vs. group 2, which may reflect that after a certain time, when patients are approaching stability, their CXCL10 starts to plateau. We have previously shown that CXCL10 in active vitiligo patients (VIDA 3 and 4) have a statistically higher CXCL10 compared to stable (VIDA 0 and -1) vitiligo and that serum level correlated with tissue CXCL10 level [24]. Moreover, Wang et al. [25] reported that CXCL9 and CXCL10 are not only elevated in the serum of a large cohort of patients with vitiligo compared with healthy controls, but their concentrations are higher in patients with progressive vitiligo compared with those with stable disease.
This finding urges us to explore CXCL10 in patients with different durations of disease activity and/or to look for other more sensitive serum markers for disease stability like CXCL9, CD25, CD27, S100 B and others [25-28].
In patients with short duration of stability (group 1), the higher the CXCL10 level, the worse the outcome and extent of pigmentation. This highlights the role of IFN-γ and related products in melanocyte jeopardy from a direct and immune-mediated aspects [29,30], the higher they are, the more endangered are the melanocytes. On the basis of our findings, it is proposed that measuring the serum level of CXCL10 in patients with disease stability of less than one year duration could be used as a marker for the disease stability and a predictor for the results of the minigraft and probably other types of vitiligo surgery.
A certain critical level of serum CXCL10 should not be exceeded to get a good surgical outcome. ROC analysis indicated that a level below 52 ng/L may guarantee a good MGT outcome. Further studies to validate such a cut-off value using the same ELISA kits and same methodology are advocated. We have previously found a 100 ng/L cutoff level between active and stable cases, CXCL10 was the most specific (96.00% specificity, 83.33% sensitivity), while IL-6 was the most sensitive marker (80.00% specificity, 96.67% sensitivity) for disease activity [24]. Assumingly, the cut off level for stability is different from that for a good MGT outcome. Alternatively, cut off levels may be different when using different kits and methodologies.
Our current findings indicate that MGT remains the gold standard for predicting disease stability before proceeding to larger more exhaustive surgeries, like melanocyte keratinocyte transplantation and suction blister grafting. CXCL10 level may be helpful in patients with shorter disease stability wishing for surgical interference.
Citation: Abdallah M, Afify AA, Asaad MK, Hassan RMA (2019) Evaluation of Serum CXCL10 in Relation to Minigraft Test in Detecting Stability of Vitiligo. J Clin Exp Dermatol Res 9: 491. doi:10.4172/2155-9554.1000491
Received: 13-Feb-2019 Accepted: 14-Mar-2019 Published: 21-Mar-2019
Copyright: © 2019 Abdallah M et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Sources of funding : GB´s PhD-project on ethical challenges and decision-making in nursing homes has been financially supported by the Norwegian Extra Foundation for Health and Rehabilitation through EXTRA funds (grant no. 2008/2/0208).