Journal of Plant Biochemistry & Physiology
Open Access
ISSN: 2329-9029
ISSN: 2329-9029
Research Article - (2019)Volume 7, Issue 4
Cyanide remediation potential of plants is due to their ability to synthesize cyanogenic glucosides. Naturally, cyanogenic plants not only synthesize organic cyanides but are also imbue with efficient degradation potentiality. Example of such plants is Chromolaena odorata which is widely known to be effectively used as a therapy against several ailments. Recently, the plant has been employed in the remediation of cyanide from contaminated sites. Cyanide intoxication mediate pathologic effects on different tissues that precede alterations in biochemical parameters. The present study was aimed to evaluate the effects of sublethal cyanide exposure and ameliorative effects of sodium thiosulphate and ethanol Extract of Chromolaena odorata (ECO) administered singly and in combination on some enzyme activities in rats. Thirty five male rats were divided into seven groups. All test groups received potassium cyanide (KCN) at 7 mg/kg body weight; Control group:received distilled water daily for the experimental period. Cyanide group: received KCN at 7.0 mg/kg administered via gavage; KCN+100ECO group:received KCN and 100 mg/kg of ECO; KCN+150ECO group:received KCN and 150 mg/kg of ECO; KCN+200ECO group:received KCN and 200 mg/kg of ECO; KCN+Na2S2O3 group:received KCN and oral administration of sodium thiosulphate at 200 mg/kg; KCN+ECO+Na2S2O3 group:received KCN and oral administration of both sodium thiosulphate and ECO at 200 mg/kg. At the end of the experiment, activities of Rhodanese, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and superoxide dismutase were measured. Potassium cyanide administration caused alteration in the measured enzymes while sodium thiosulphate and Chromolaena odorata ethanol extracts relieved the alterations. Whereas, combination of the two regimen showed a synergistic effect. The altered tissue activities of some enzymes in the present study might reflect the metabolic disturbances due to cyanide intoxication. However, further research should be focused on this issue for better understanding of the fine mechanism of cyanide effects upon metabolic enzyme activities.
Cyanide; Chromolaena odorata; Sodium thiosulphate; Rats
Cyanide is a noxious chemical well-known for its characteristics as suicidal, homicidal and chemical warfare agent [1]. It is generated in ample quantities from metal plating and finishing, mining and extraction of metals such as gold and silver, production of synthetic fibers and the processing of coal [2]. However, discharge of cyanide containing wastes into the environment has birth considerable interest [3]. Cyanide is found to be highly poisonous to living organisms, primarily due to the formation of complexes with metal ions that are present as enzyme cofactors. The notable effect of cyanide occurs with Fe3+ ion in cytochrome, thereby obstructing cellular respiration and hence, oxidative phosphorylation [4].
Biochemical alterations are considered as sensitive indicators of toxicity before the expression of visible hazardous effects. Biochemical screening has been embraced to provide an early warning of potentially harmful changes in stressed organisms. For example, evaluation of intermediary metabolism of fish was used, to study cyanide mediated effects [5,6]. Under stress, enzymatic activities are subjected to changes. Toxicants initiate stress response in an organism which can be observed via changing enzymatic activity [7]. Enzymatic activity appears to be altered due to the active site being either denatured or distorted when an organ-system is diseased due to the effect of a toxicant. Since some enzymes catalyze metabolic pathways they are ubiquitous in the biological system. It is suffice to say that alterations in their levels may be sufficient to provide information of diagnostic values [8].
Response to stress impose some changes with biochemical and physiological relevance which may either be acute or chronic [9]. The disruption of biochemical and physiological integrity is assayable by the monitoring changes in the enzyme activities in functional organs. Metabolism brings together several elements including enzyme activity and modulation, substrate pools and physiological response. Therefore, in addition to inhibition of cytochrome c oxidase, the toxicity of cyanides can be sensitively recognized as a measure of metabolism. Moreover, Protein represents the most important and abundant biochemical component of the living system. Due to the abundant and essential role it serves, understanding protein metabolism becomes crucial even in trivial changes taking place in its profile during cyanide poisoning. Both the buildup and breakdown of protein influenced by a wide range of conditions. Several physical and chemical modulators can impose colossal loss of activity. Cellular dysfunction mediated by stress can be detected via structural and functional changes of cellular proteins. Most enzymes are protein in nature and so the importance of proteins in metabolism cannot be underestimated [10].
Alterations in enzymatic profiles have been employed as a useful index in pollution studies. It was explained that changes in the enzymatic activities of the fish intoxicated with cyanide is due to increased permeability of the cell, as well as direct effect of cyanides on the tissues [9]. Transamination is one of the principal pathways for the biosynthesis and deamination of amino acids, enabling interchange between carbohydrate and protein metabolism during changing energy demands [11]. It was posited that the maintenance of internal homeostasis through biochemical events in the Tricarboxylic acid cycle may be indicated in varying levels of the enzymes engineered by cellular damage in the functional organs [12]. There is hike in the serum levels of both aspartate aminotransaminase (AST) and alanine aminotransaminase (ALT) when disease process affects cell integrity and ALT being more liver-specific.
Inorganic cyanides are extremely toxic, which might hamper the health status through the impairment in the metabolism, sometimes leading to death. This is because, most of the cyanide absorbed is detoxified by enzymatic combination with sulfur, and thus the detoxification process imposes a nutritional cost [5]. Concentrations of cyanides as well as lethal and sublethal toxicities of cyanides to the animals have been well documented [13]. Evaluation of biochemical activities in an organism helps to identify disturbances in its metabolism. In this study, we monitored the disturbances of metabolism in rats by exposing them to sublethal concentrations of potassium cyanide for a period of 14 days.
Plant materials (collection and preparation)
Stem with Leaves of C. odorata were collected from an uncultivated land near a residential location at Hamama area, Ogbomoso, South-Western Nigeria and identified by Prof. A.T.J Ogunkunle at the Herbarium of Ladoke Akintola University of Technology (LAUTECH), Ogbomoso with voucher number, LHO 526. They were air dried, packed in paper bags and stored. The dried leaves were pulverized and 196 g of the pulverized sample was extracted with 500 ml of 80% ethanol by maceration for 72 h. The ethanolic extract was concentrated in a rotary evaporator, lyophilized and thereafter preserved for further use.
Experimental procedure
Thirty-five (35) male albino rats weighing between 100 g and 150 g were distributed randomly into seven (6) experimental groups and One (1) control group.
Control group: Received distilled water daily for the experimental period.
Cyanide group: Received KCN solution at concentration 7 mg/kg administered via gavage; the amount of KCN administered was adjusted to the body weight.
KCN+100ECO group received KCN and 100 mg/kg ethanol extract of Chromolaena odorata (ECO) group.
KCN+150ECO group received KCN and 150 mg/kg ethanol extract of Chromolaena odorata (ECO) group.
KCN+200ECO group received KCN and 200 mg/kg ethanol extract of Chromolaena odorata (ECO) group.
KCN+Na2S2O3: received KCN and oral administration of sodium thiosulphate at 200 mg/kg.
KCN+ECO+Na2S2O3 group: received KCN and oral administration of both sodium thiosulphate and ECO at 200 mg/kg.
The animals, which were from the same litter, were acclimatized for two weeks. They were fed on commercial rat pellets and water ad-libitum. They were kept in cages whose dimensions are 30 cm by 15 cm by 25 cm at room temperature. The study was conducted at the Animal House in the department of Biochemistry, Ladoke Akintola University of Technology, Ogbomoso, Oyo State, Nigeria.
After a brief exposure of the rat to diethyl ether (anesthetic) making the animal unconscious, the animals were placed on a dissecting board with the limbs pinned to the board. The rat is dissected starting from the central abdominal region upward to expose the internal region and organs. Using a syringe and needle, the blood was collected from the heart of each rat, centrifuged to obtain sera used for biochemical analyses. Liver, Kidney and brain samples were removed, quickly washed in ice cold sucrose solution, blotted with filter paper and weighed. A 10% whole tissue homogenate in phosphate buffered saline (pH 7.4) was prepared and centrifuged at 10,000 g at 4°C for 10 min to obtain the supernatant which was used for biochemical analyses.
Enzyme assays
Rhodanese, Alanine aminotransferase (ALT), Alkaline Phosphatase (ALP), Superoxide anion dismutase (SOD), Glucose 6-phosphate dehydrogenase (G6DPH), Lactate dehydrogenase (LDH) activities were determined using commercial kits and following standard procedures outlined by the producer, Randox Laboratories, UK.
Statistical analysis
All calculations were performed using SPSS/PC software. All results were analyzed using one way analysis of variance (ANOVA), followed by Duncan's multiple comparisons test. The level of significance was set at p<0.05.
Effects of KCN, ECO and Na2S2O3 on hepatic rhodanese activity
Expressed in Figure 1 below are the results of the liver rhodanese activities. Cyanide-only-group liver rhodanese activity was not significantly increased with respect to the control group (p>0.05). This difference was observed to increase for all therapeutic groups with respect to the control. Only during administration of cyanide+Na2S2O3 and co-administration with the extract, liver rhodanese activity was significantly higher with respect to the control and KCN-only groups.
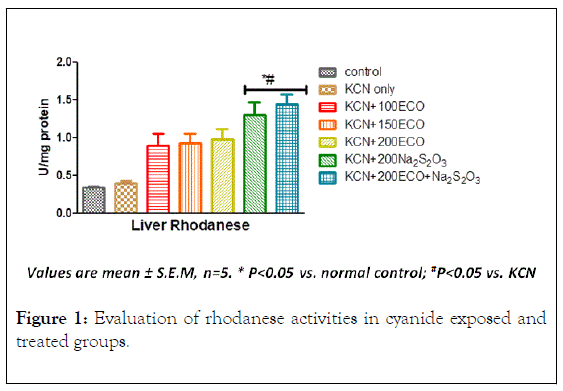
Figure 1: Evaluation of rhodanese activities in cyanide exposed and treated groups.
The slight increase in the activity of this enzyme in the presence of cyanide may be due to the induction of the enzyme by this toxic substance and the increase observed when Na2S2O3 was co-administered may be an additional effect produced as a result of increased availability of sulphur supplied by Na2S2O3. The results of the study carried out by Ehigie et al. 2019 [14], which shows that rhodanese has preference for sodium thiosulphate among different sulphur compounds tested namely Mercapto- (HOCH2CH2SH), Ammonium per sulfate (NH4)2S2O8), Ammonium sulfate (NH4)2SO2), Sodium sulfate (Na2SO4), Sodium metabisulfate (Na2SO5) informed the choice of sodium thiosulphate used in this study. Since the major mechanism for the excretion of cyanide is its enzymatic conversion by the enzyme rhodanese to thiocyanate (SCN), increased activity of this enzyme observed in the extract and thiosulphate groups indicates increased detoxification of cyanide by this pathway. It has been postulated that the observed abundance of potentially functional rhodanese-like proteins may suggest that members of this homology superfamily may play distinct biological roles [15]. Rhodanese play a variety of functions ranging from transport mechanisms of sulfur⁄selenium in a biologically available form [16], to the modulation of general detoxification processes [17] and to the restoration of iron-sulfur centres in Fe-S proteins, either alone or in combination with other proteins. These roles may be mediated by Rhodanese alone or in combination with other proteins. Many studies have it proved that the rhodaneselike proteins have roles in ‘managing’ stress tolerance and in maintaining redox homeostasis [18]. Microarray studies have revealed that decrease expression of rhodanese gene is associated with the incidence of carcinogenesis [19]. In fact, sensitivity of tumor cells to cytotoxic organosulphur compounds as a result of the decreased rate of clearance of these compounds is due to the down-regulation of rhodanese in such cells [20].
Effect of KCN, ECO and Na2S2O3 on Lactate dehydrogenase (LDH) and Glucose 6-Phosphate dehydrogenase (G6DPH) activities
KCN significantly increased the activities of LDH and G6PDH with respect to control groups (Figure 2). All treatment groups demonstrated significant decrease (p<0.05) in LDH activity relative to group administered only potassium cyanide. Under G6DPH, only groups administered with Na2S2O3 and ECO combined with Na2S2O3 showed significant increase in G6DPH activity.
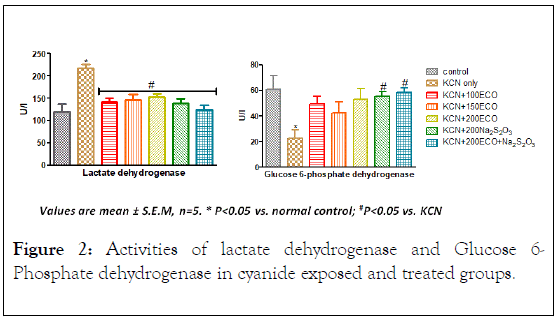
Figure 2: Activities of lactate dehydrogenase and Glucose 6- Phosphate dehydrogenase in cyanide exposed and treated groups.
LDH, the terminal enzyme in vertebrate anaerobic glycolysis, is one of the enzymes that have been employed for diagnosing liver damage. LDH is an oligomeric enzyme recognized as a potential marker for assessing the chemical toxicity [21]. Increase in LDH activity in the present study may be due to inhibition of cytochrome oxidase activity [22]. This situation might be as a result of the shift from aerobic to anaerobic respiration due to the mild stress of hypoxia; thus, aerobic processes might be operating at a very slow rate. Similar observations were made by David et al. [23] in the fish C. carpio, exposed to sodium cyanide. Increase in LDH activity is substantiatied by conversion of pyruvate to lactate at the expense of NAD. Consequentially, to fulfill the energy demands, there may be increase in the operation of carbohydrate metabolism under cyanide stress [24]. Changes in intermediary metabolism due to stress results in altered activity of oxidative enzymes like SDH, LDH, and G6PDH. These may also contribute to the classic signs of cyanide toxicity [25].
Stimulation of LDH under cyanide intoxication portends disturbances in the cellular oxidative processes [4]. The changes appear to favor alternative metabolism in response to the exogenous stress, probably due to low utilization of oxygen for the normal metabolic functions. Increased LDH resulted in lactic acidosis, due to an enhanced rate of conversion of pyruvate to lactate [24,26]. Similarly, under hypoxic condition, augmented release of LDH in to tissues was reported by Sharma and Jain [27]. The significant increase of LDH activity observed in this study suggests the decrease in the glycolytic process due to the increased metabolic rate as a result of cyanide exposure [22].
Glucose-6-Phosphate dehydrogenase (G6PDH) is a key enzyme that catalyses the oxidative irreversible step in Pentose phosphate pathway and exhibits decreased activity under cyanide intoxication [28]. G6PDH is mainly regulated by the NADPH/ NADP ratio [29], needed as reducing power for various detoxification pathways. The effects of different chemical substances on the activity of G6PDH enzyme have been investigated in many in vitro and in vivo studies, performed with various organisms [30]. In the present study, potassium cyanide significantly altered G6PDH activity in rats indicating defects in mobilization of glucose through pathways other than glycolysis- Krebs cycle. Changes in the dehydrogenase activity in cyanide treated animal tissues may be due to fluctuations in oxidative metabolism [31]. The significant decrease in the activity of G6PDH observed during cyanide poisoning suggests the defect in NADPH generation via HMP shunt which predisposes the red blood cells to oxidative damage by free radicals. NADPH is a coenzyme for the recycling of reduced Glutathione in red blood cells. The increase in G6PDH activity may suggest a possible regulatory role played by rhodanese [32]. In fact, Rhodanese has been described to mediate the regeneration of reduced Glutathione [33].
The activities of Superoxide dismutase in the liver, kidney, and brain of rats exposed to sublethal dose of KCN
Lower rhodanese protein expression (Figure 3) has been shown to be significantly associated with higher mitochondrial superoxide production and lipid peroxidation [34] confirming the hypothesis that impaired expression of the thiosulfate sulfurtransferase (rhodanese) is associated with oxidative stress.
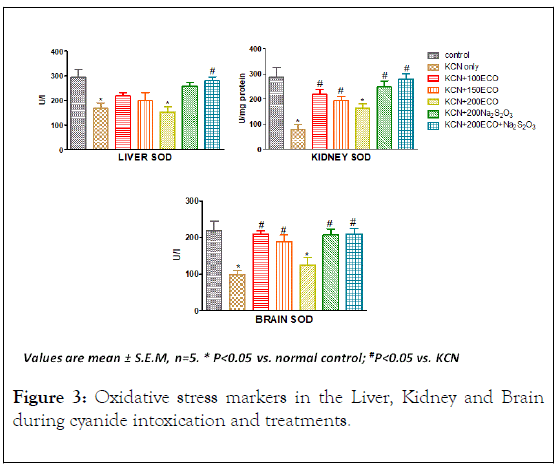
Figure 3: Oxidative stress markers in the Liver, Kidney and Brain during cyanide intoxication and treatments.
SOD activity in all tissue examined was significantly decreased under group administered only KCN. In the liver, only ECO +Na2S2O3 revealed significant increase in SOD activity relative to KCN-only group. In the Kidney, and Brain tissues, there was significant increase in SOD activities in the therapeutic groups relative to KCN-only group except for 200 mg/kg ECO group. The decreased activity of SOD in the liver, kidney and brain tissues of cyanide treated rats may be due to increased utilisation of the enzyme against reactive oxygen species [35]. Oxidative stress ensues when the trade-off between oxidants and antioxidants is compromised due to the depletion of antioxidants or excessive accumulation of the reactive oxygen species, or both, leading to cellular damage [36].
Antioxidants, protects cells from dysfunction mediated by reactive species by absorbing the reactive oxygen species prior to their interaction with cellular components thus making them the first line of defence against exogenous oxidative stress [37].
It has been posited that Rhodanese function as an antioxidant protein prevents oxidative stress as a result of its interaction with a number of endogenous molecules such as Glutathione, Thioredoxin forming a system which maintains redox homeostasis [33]. The relieved oxidative stress observe in the therapeutic groups could be as a result of the modulation of Rhodanese activity.
Effects on L–Aspartate Aminotransferase (AST) and LAlanine Aminotransferase (ALT)
The activities of Alanine aminotransferase (ALT) and Aspartate Aminotransferase (AST) in the serum and liver of exposed to cyanide are shown in Figure 4. In the Liver only group treated with potassium cyanide only showed significant decrease in the activity of AST relative to the control group. However, groups dosed with KCN, 100 mg/kg ECO, 150 mg/kg ECO and 200 mg/kg ECO showed significant decrease in the activity of ALT relative to the control group. The groups dosed with sodium thiosulphate alone and in combination with 200 mg/kg ECO showed significant increase (p>0.05) in the activity of Liver ALT with respect to the KCN-only group. In the serum, there was significant increase in the activities of AST and ALT in groups dosed with KCN-only and 200 mg/kg ECO relative to control group. However, the increase in the activities of ALT and AST was abated significantly (p<0.05) by other groups.
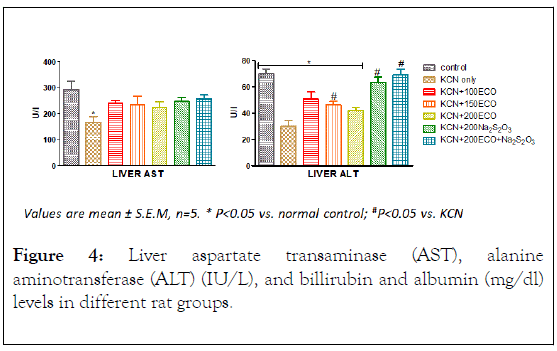
Figure 4: Liver aspartate transaminase (AST), alanine aminotransferase (ALT) (IU/L), and billirubin and albumin (mg/dl) levels in different rat groups.
Transamination is one of the principal pathways for the synthesis and deamination of amino acids, enabling carbohydrate and protein metabolism to be sustained during fluctuating energy demands under various adaptive conditions [38].
Maintenance of internal homeostasis through biochemical processes in the Krebs’s cycle may be reflected in increased levels of the transaminases. Thus it is conceivable that the decreased activity of ALT and AST in the liver and corresponding increase in the serum of cyanide exposed rats is an indication of Liver damage orchestrated by cyanide.
Effects on Alkaline Phosphatase
Significant increase was observed in the serum activity of Alkaline Phosphatase enzyme during cyanide exposure (Figure 5). KCN-only group significantly increased ALP activity when compared to the control group. The increase in ALP activity was significantly reduced (p<0.05) by all the extract and sodium thiosulphate dosed groups except for the groups administered 200 mg/kg ECO which showed no significant difference relative to the groups administered KCN only.
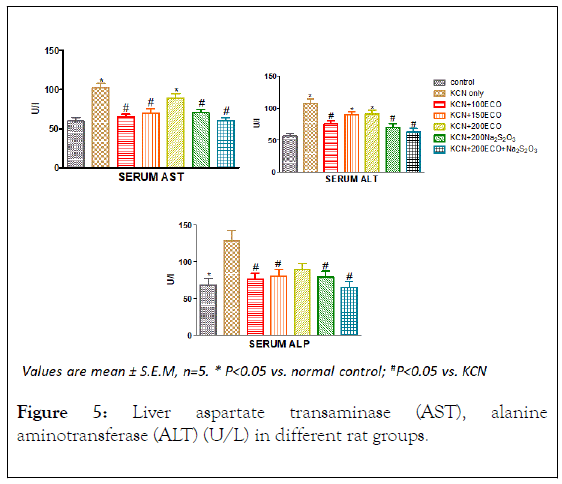
Figure 5: Liver aspartate transaminase (AST), alanine aminotransferase (ALT) (U/L) in different rat groups.
The increased activity of ALP in the serum may be due to the de novo synthesis of the enzyme molecule. ALP is a marker enzyme for the plasma membrane and endoplasmic reticulum (Wright and Plummer) of the tissues. It is often employed to assess the integrity of the plasma membrane [39] since it is localized predominantly in the microvilli in the bile canaliculli, located the plasma membrane. Since ALP hydrolyses phosphate monoesters the enzyme’s hyper production could constitute a threat to the life of the cells that are dependent on a variety of phosphate esters for their vital process [40] as it may lead to indiscriminate hydrolysis of phosphate ester metabolite of the liver. Consequently this may adversely affect the facilitation of the transfer of metabolites across the cell membrane. It follows therefore that such hyper production of ALP may also have severe consequences on the architecture of the cells of affected organs.
The results of this study clearly shows disruption in enzymatic activities in rats exposed to potassium cyanide and this may lead to a severe energy crisis at the cellular level and altered the intermediary metabolism. Decreased enzymatic activities in various tissues under cyanide intoxication indicate the switch over of metabolic pathways towards compensatory mechanisms. Therefore, alterations in the intermediary metabolism reflects the differential effects of stress and can be considered as a diagnostic tool in the assessment of cyanide poisoning in living organisms.
Citation: Ojeniyi FD, Ehigie AF , Ehigie OL (20 1 9) Evaluation of Enzymatic Changes in Suble thal Cy anide P oisoning Wis t ar Rats T r eat ed with Chromolaena odorata (Linn.) and Sodium Thiosulphate. J Plant Biochem Physiol. 7:242. DOI: 10.35248/2329-9029.19.7.242
Received: 10-Dec-2019 Accepted: 24-Dec-2019 Published: 31-Dec-2019
Copyright: © 2019 Ojeniyi FD , e t al. This is an open-access ar ticle dis tribut ed under the t erms of the Cr eativ e Commons A ttribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.