Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2020)Volume 11, Issue 4
Background: Available treatments for acne vulgaris are associated with various adverse effects, which necessitate
patients to opt for alternative treatment options.
Objectives: To evaluate efficacy and safety of AHPL/AYCAP/0413 capsule and AHPL/AYTOP/0213 cream in
subjects suffering from Acne Vulgaris.
Method: A total of 62 subjects completed the study. Subjects were advised to take 2 capsules of AHPL/AYCAP/0413
twice daily orally after meals with water and apply AHPL/AYTOP/0213 cream all over the face twice a day for two
months or complete resolution of acne whichever was earlier. The primary objective was to assess changes in total
number of inflammatory acne lesions on face. Secondary objectives were to assess changes in total numbers of noninflammatory
acne lesions, total lesion count (inflammatory and non-inflammatory), acne severity, acne scarring,
signs and symptoms, skin lightening effect, post acne dark spots, adverse events and laboratory investigations.
Results: The mean inflammatory acne lesion count reduced significantly from 7.94 ± 5.91 (baseline visit) to 1.89 ±
3.06 (p=0.001) at the end of treatment. Significant reduction in total numbers of non-inflammatory acne lesions,
total lesion count, acne severity and signs and symptoms of acne were observed at the end of treatment. Also
improvement in skin colour, severity of post acne dark spot and reduction in numbers of post acne dark spots were
observed at the end of treatment. Laboratory parameters were within normal limits both at pre and post treatment.
Conclusion: AHPL/AYCAP/0413 capsule and AHPL/AYTOP/0213 cream is safe and significantly effective for
treatment of acne vulgaris.
Acne vulgaris; AHPL/AYCAP/0413; AHPL/AYTOP/0213
Acne is that common clinical condition for which patients seek Dermatological care [1]. Topical antibiotics like clindamycin, erythromycin and anti-inflammatory medicines like retinoids and adapalin are effectively used in the treatment. Dryness, erythema, burning, local irritation and photosensitivity are common side effects observed with the use of Retinoids, which are highly effective otherwise.
Oral antibiotics i.e. tetracycline, erythromycin, doxycycline and minocycline are used. Oral contraceptive pills and oral retinoids are also used in the systemic treatment of acne. Tetracycline, Doxycycline may lead to adverse effects like gastric disturbances, tinnitus, vertigo, discoloration of the teeth. Minocycline is associated with lupus erythematous. The risk of autoimmune reactions increases with the duration of use. The use of Retinoids has to be done with caution as they are said to be teratogens. Cheilitis, dry skin, elevated liver transaminase levels, hypertriglyceridemia and decreased night vision are common adverse effects associated with Retinoids [2].
Keeping in mind the basic concept of Ayurveda, Ari Healthcare Pvt. Ltd., has developed AHPL/AYCAP/0413 capsule (Aricleanse Capsule) and AHPL/AYTOP/0213 cream (Aricleanse Cream) for effective management of Acne vulgaris. The composition of the drugs is given in Tables 1 and 2. Study drugs contain seven and eight ingredients respectively that help in regulation of excessive production of sebum, and relieving the inflammation of lesions of acne vulgaris. Looking at the activities of the ingredients of study drugs, a hypothesis was postulated that study drugs will be helpful in the management of Acne vulgaris. Hence, to test this hypothesis, a clinical study titled “Evaluation of Efficacy and Safety of AHPL/AYCAP/0413 capsule and AHPL/AYTOP/0213 cream in patients suffering from Acne Vulgaris” was planned.
| Sr. No. | Ingredients | Latin Name | Quantity |
|---|---|---|---|
| 1 | Sariva root extract | Hemidesmus indicus | 100 mg |
| 2 | Guduchi stem extract | Tinospora cordifolia | 100 mg |
| 3 | Manjishtha stem extract | Rubia cordifolia | 70 mg |
| 4 | Yashtimadhuka stem/root extract | Glycyrrhiza glabra | 60 mg |
| 5 | Nimba leaf extract | Azadirachta indica | 50 mg |
| 6 | Khadira bark extract | Acacia catechu | 50 mg |
| 7 | Kakamachi whole plant extract | Solanum nigrum | 50 mg |
Table 1: Each AHPL/AYCAP/0413 Capsule Contains.
| Sr. No. | Ingredients | Latin Name | Quantity |
|---|---|---|---|
| 1 | Lodhra bark extract | Symplocos racemosa | 3% w/w |
| 2 | Vacha root extract | Acorus calamus | 3% w/w |
| 3 | Dhanyaka fruit extract | Coriandrum sativum | 3% w/w |
| 4 | Yashtimadhuka stem/root extract | Glycyrrhiza glabra | 2% w/w |
| 5 | Shalmali exudate extract | Salmalia malabarica | 3% w/w |
| 6 | Daruharidra root extract | Berberis aristata | 3% w/w |
| 7 | Jatiphala fruit extract | Myristica fragrans | 2% w/w |
| 8 | Manjishtha stem extract | Rubia cordifolia | 2% w/w |
Cream base: QS to make 100%
Table 2: Each gm of AHPL/AYTOP/0213 Cream contains% of ingredients (w/w).
This was an open label, interventional, single arm, single center, prospective clinical study, conducted at MAM's SS Ayurveda Mahavidyalaya and Sane Guruji Aarogya Kendra, Malwadi, Hadapsar, Pune-411028. The study was approved by Institutional ethics committee. The study was conducted in accordance with Good clinical practices (GCP) guidelines (issued by AYUSH in 2013). Also the study is registered (CTRI/2016/06/007032, dated 23/06/2016) on clinical trial registry of India.
Male and female patients between 18 to 40 years of age suffering from acne vulgaris and who signed the informed consent form were recruited in the study.
Patients with preexisting systemic disease necessitating long-term medications, history of genetic and endocrinal disorders, patients who had menstrual disorders and patients with drug induced acne, patient suffering from major/severe illness(s), pregnant and lactating women, patients with significant abnormal laboratory parameters, and ECG demonstrating any signs of uncontrolled arrhythmia were excluded from the study.
Each study subject had a total of 6 visits viz. Screening Visit (day -7), baseline visit (Day 0), Visit 1 (Day 15), Visit 2 (Day 30), Visit 3 (Day 45) and Visit 4 (Day 60).
On screening visit, written informed consent was obtained from subjects. Severity of acne vulgaris was assessed using Global Acne Grading System (GAGS). Subjects having severe nodular or cystic acne were not recruited in the study. Subject underwent investigations like ECG, fasting blood sugar, CBC, Hb%, ESR, Liver function tests, Renal function tests, Lipid profile, Urine routine and microscopic, X-ray Chest (PA view), Urine pregnancy test (only if the subject was female of child bearing potential) and HIV I and II tests.
Eligible subjects were recruited in the study. Subjects were advised to take 2 capsules of AHPL/AYCAP/0413 twice daily orally after meals with water and apply sufficient quantity of AHPL/AYTOP/0213 cream all over the face, twice a day after washing face with lukewarm water for 60 days or complete resolution of acne, whichever was earlier. Drug compliance was checked on each follow up visit.
On every follow up visit, subjects underwent general and systemic examinations. All the subjects were closely monitored for any adverse events on each visit. All the assessments regarding the primary and secondary objectives were conducted on each visit. On final follow up visit or on the day of complete resolution of acne (as per investigator ’ s opinion), patient ’ s laboratory investigations viz. CBC, ESR, Hb%, Liver function tests, Renal function tests, Lipid profile, Urine routine and microscopic and ECG were done. Patient’s global evaluation and Investigator’s global evaluation for overall improvement and tolerability of trial medicines were performed on the last follow up visit.
Change in total number of inflammatory acne lesions on face was the primary endpoint of the study. Secondary endpoints were to assess changes in the total numbers of noninflammatory acne lesions, changes in total lesion count (inflammatory and non-inflammatory), changes in acne severity assessed using Global Acne Grading System (GAGS), changes in acne scarring score (Goodman ’ s quantitative global acne scarring grading system), changes in signs and symptoms, skin lightening effect on a graded scale, changes in post acne dark spots (numbers and severity on graded scale), patient’s global evaluation and investigator ’ s global evaluation for overall improvement, tolerability of study drugs, adverse events and changes in laboratory investigations.
The largest or prominent post acne dark spot was considered as index spot (for evaluation of effect of test drugs) and the same was assessed till the completion of the study with six-point Likert scale.
Statistical analysis was performed using statistical software SPSS 10.0. Efficacy analysis was performed by using student t-test, Chi square test, summary statistics. All p-values were reported based on two-sided significance test and all the statistical tests were interpreted at 5% level of significance. Safety analysis was done by assessing the adverse events (AE) and adverse drug reactions (ADR). Any clinically significant changes in laboratory parameters were also reported.
A total of 86 subjects suffering from Acne vulgaris were screened for recruitment in the study. 6 patients did not fulfil the inclusion /exclusion criteria hence were not enrolled in the study. A total of 80 subjects were recruited in the study. Out of 80 recruited subjects, 62 completed the study, while 18 subjects dropped out prematurely.
The reason for drop out was lost to follow up (17 subjects) and request to discontinue (1 subject). Completed subjects were in the age range of 18 to 40 years, the mean age was 24.19 years.
Out of 62 subjects, 18 were males (45.2%) and 34 were females (54.8%). The mean height of the subjects was 163.55 cm and mean weight was 58.57 kg.
At baseline visit, the mean inflammatory acne lesion count was 7.94 ± 5.91, which reduced significantly from day 15 onwards and this trend continued till the end of the treatment (Day 60) when the count was 1.89 ± 3.06.
The analysis demonstrates that 43.5% subjects did not have a single inflammatory lesion on the completion of treatment. The details are presented in Table 3, and Figure 1.
| Duration in Days | Mean inflammatory lesion count (X͞ ± SD) (N=62) |
|---|---|
| Baseline | 7.94 ± 5.91 |
| 15 | 5.21 ± 5.37 |
| 30 | 3.95 ± 4.76 |
| 45 | 2.66 ± 3.01 |
| 60 | 1.89 ± 3.06 |
| Mean diff. (Baseline-15 ) (p Value) |
*-2.73 ± 03.15 (0.001) |
| Mean diff. (Baseline-30 ) (p Value) |
*-3.99 ± 03.40 (0.001) |
| Mean diff.(Baseline-45 ) (p Value) |
*-5.28 ± 4.73 (0.001) |
| Mean diff. (Baseline-60 ) (p Value) |
*-6.05 ± 4.75 (0.001) |
By student t test, *Significant
Table 3: Mean inflammatory lesion count.
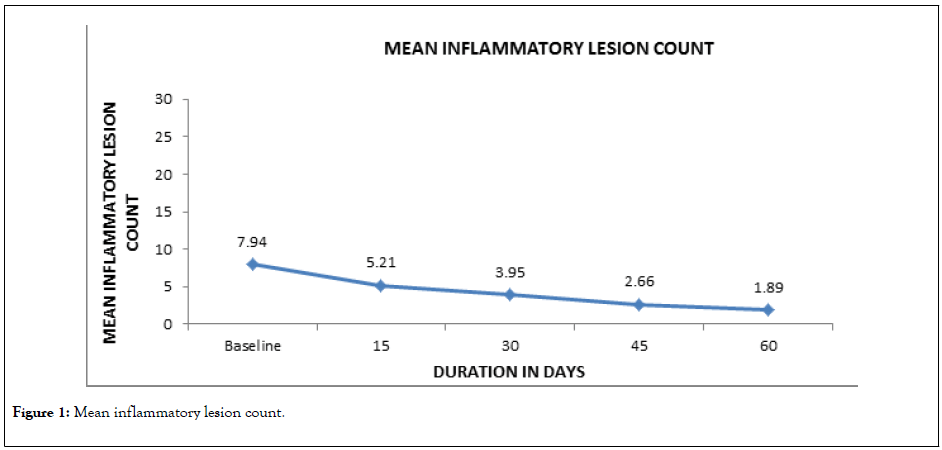
Figure 1: Mean inflammatory lesion count.
On baseline visit, the mean non-inflammatory acne lesion count was 39.37 ± 38.30, which reduced significantly from day 15 onwards and continued till end of the treatment.
The count reduced to 19.69 ± 20.05 at the end of the treatment. The details are given in Table 4 and Figure 2.
| Duration in Days | Mean non-inflammatory lesion count (X͞ ± SD) (N=62) |
|---|---|
| Baseline | 39.37 ± 38.30 |
| 15 | 31.63 ± 28.50 |
| 30 | 27.40 ± 24.12 |
| 45 | 25.48 ± 24.11 |
| 60 | 19.69 ± 20.05 |
| Mean diff. (Baseline-15 ) (p Value) |
*-07.74 ± 16.73 (0.001) |
| Mean diff. (Baseline-30 ) (p Value) |
*-11.97 ± 22.37 (0.001) |
| Mean diff. (Baseline-45 ) (p Value) |
*-13.89 ± 28.10 (0.001) |
| Mean diff. (Baseline-60 ) (p Value) |
*-19.68 ± 30.14 (0.001) |
By student t test, P<0.05, *Significant
Table 4: Mean non-inflammatory lesion count.
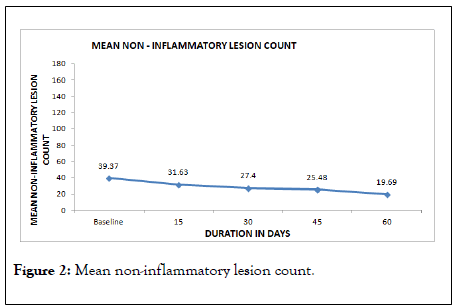
Figure 2: Mean non-inflammatory lesion count.
On baseline visit, the mean total lesion count (Inflammatory and non-inflammatory) was 47.31 ± 40.43. Significant fall (p=0.001) in the mean total lesion count was observed from day 15 and this trend continued till the end of the treatment when the count was 21.58 ± 21.04. The details are presented in Table 5 and Figure 3.
| Duration in Days | Mean total lesion count (X͞ ± SD) (N=62) |
|---|---|
| Baseline | 47.31 ± 40.43 |
| 15 | 36.84 ± 30.58 |
| 30 | 31.35 ± 25.62 |
| 45 | 28.15 ± 25.39 |
| 60 | 21.58 ± 21.04 |
| Mean Diff. (Baseline-15 ) (p Value) |
*-10.47 ± 16.90 (0.001) |
| Mean Diff. (Baseline-30 ) (p Value) |
*-15.96 ± 22.86 (0.001) |
| Mean Diff. (Baseline-45 ) (p Value) |
*-19.16 ± 29.00 (0.001) |
| Mean Diff. (Baseline-60 ) (p Value) |
*-25.73 ± 31.00 (0.001) |
Table 5: Mean total lesion count.
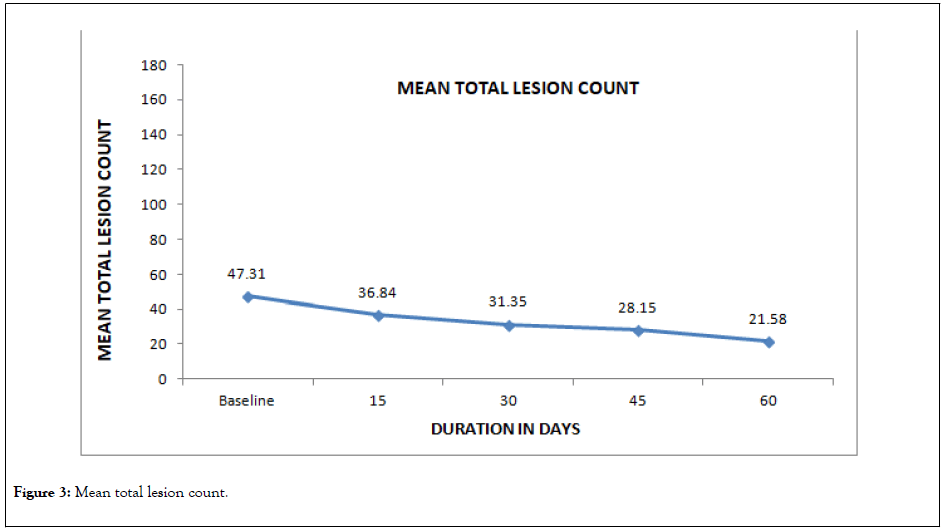
Figure 3: Mean total lesion count.
The mean global score of acne severity was 14.79 ± 2.86 on the baseline visit. Statistically significant (0.001) reduction in the mean global score of acne severity was observed from day 15 and continued till the end of the treatment. At the end of the treatment, the mean global score of acne severity was 8.89 ± 3.42 indicating significant fall of 39.9% from baseline visit. On completion of the study period, not a single subject had acne severity of moderate grade. The details are presented in Table 6.
| Duration in days | Mean global score of acne severity (X͞ ± SD) (N=62) |
|---|---|
| Baseline | 14.79 ± 2.86 |
| 15 | 11.90 ± 2.77 |
| 30 | 10.85 ± 3.44 |
| 45 | 10.13 ± 3.32 |
| 60 | 8.89 ± 3.42 |
| Mean diff. (Baseline-15 ) (p Value) |
*-2.89 ± 2.75 (0.001) |
| Mean diff. (Baseline-30 ) (p Value) |
*-3.94 ± 3.13 (0.001) |
| Mean diff. (Baseline-45 ) (p Value) |
*-4.66 ± 3.41 (0.001) |
| Mean diff. (Baseline-60 ) (p Value) |
*-5.90 ± 3.19 (0.001) |
Table 6: Mean global score of acne severity assessed on GAGS.
On baseline visit, 1.6% subjects had severe pain due to acne lesions, 25.8% had moderate pain and 58.1% had mild pain while 14.5% did not experience any pain. At the end of the study, 74.2% cases had no pain, 21% cases had mild pain and 4.8% cases had moderate pain.
According to the analysis, 1.6% of cases had no oedema, 62.9% cases had mild oedema and 35.5% cases had moderate oedema at baseline visit. At the end of the study, 53.2% cases had no oedema, 45.2% cases had mild oedema and 1.6% cases had moderate oedema.
On baseline visit, 40.3% subjects had moderate erythema, 53.2% had mild erythema while 6.5% subjects had no erythema. At the end of the study, 50% subjects had no erythema, 46.8% cases had mild erythema and 3.2% cases had moderate erythema at acne lesions.
Burning sensation at acne was mild in 24.2% subjects, moderate in 6.5% subjects, while 69.3% subjects did not experience burning sensation on baseline visit. At the end of the treament, 93.5% cases had no burning sensation and 6.5% cases had mild burning sensation at acne lesions.
No scaling was observed in 24.2% cases, mild scaling was observed in 62.9% cases and moderate scaling was observed in 12.9% cases at baseline visit. There were 51.6% cases with no scaling, 46.8% cases with mild scaling and 1.6% cases with moderate scaling at the end of the treatment. The change was statistically significant.
On baseline visit, 12.9% cases had moderate itching, 33.9% had mild itching and 53.2% cases had no itching at acne lesions. At the end of the treatment 91.9% cases had no itching and 8.1% cases had mild itching.
The mean total score of acne scarring on baseline visit was 2.52 ± 1.87. On day 60, the mean total score for acne scarring reduced insignificantly to 2.29 ± 1.89 (9.1%) from baseline visit.
On the baseline visit and day 15, 19.4% subjects had Von Luschan Scale rating 16-21 indicating dark intermediate or "olive skin", 79% had Von Luschan Scale rating 22-28 indicating dark or "brown" type and 1.6% had Von Luschan Scale rating 29-36 indicating Very dark or "black" type of skin. At the end of the treatment, improvement in color of the skin was observed, the number of cases with 16-21 scale rating was increased to 27.4%.
On baseline visit; 77.4% of cases had Fitzpatrick type V skin and 21.4% had type IV skin, whereas 1.6% cases had type VI skin. At the end of the study, 27.4% cases had type IV skin, while 72.6% cases had type V skin and no subject was there with type VI skin indicating improvement in skin color.
It was observed that, mean number of post acne dark spots at baseline was 24.94 ± 17.96. The mean number of post acne dark spots decreased from baseline to 22.13 ± 12.07 (11.3%) at the end of the treatment. The change was statistically insignificant.
The largest or prominent post acne dark spot out of all the post acne dark spots was decided as the Index spot on baseline visit. On Day 15, 82.3% cases had slight improvement in severity of acne dark spot, which was statistically significant. 8% cases experienced moderate improvement and 1.6% cases experienced excellent improvement but it was statistically insignificant as compared to the baseline. 8% cases reported no improvement from baseline visit. On completion of the study, 24.2% cases were reported to have completely cured index dark spot, while 40.3% had excellent improvement in the darkness severity of index spot. The change was statistically significant (p<0.05) as compared to the baseline.
As per the investigator’s assessment, 19.4% cases had excellent improvement and 46.8% cases had good improvement, 22.5% cases had satisfactory improvement, 08.1% cases had average improvement and 03.2% subjects had poor improvement in terms of overall remission of signs and symptoms of acne vulgaris at the end of the study.
As per the subjects’ global assessment 12.9% cases experienced excellent improvement, 50.0% had good improvement, 25.8% cases had satisfactory improvement, 6.5% cases had average improvement and 4.8% had poor improvement in terms of overall remission of signs and symptoms of Acne vulgaris at the end of the study.
As per the investigator ’ s as well as the subject ’ s evaluation, 98.4% cases were reported to have excellent tolerability and 1.6% had good tolerability to the study drugs.
42 subjects were reported to have at least one adverse event in the course of the 60 days of treatment period. Adverse events were mild to moderate in severity and included symptoms like fever, cough, cold, headache, vomiting etc. and were not related to the study medication. Amongst 80 recruited subjects, only 1 subject suffered from possible adverse drug reaction presented as allergic dermatitis and abdominal pain. However its association with the study drugs was doubtful as per the investigator and could not be confirmed since the subject denied the challengede- challenge procedure.
It was observed that all the laboratory investigations were within normal limits on baseline visit and at the end of the study visit. No significant post treatment change in any of the vital signs (pulse rate, body temperature, and respiratory rate, systolic and diastolic blood pressure) and body weight was observed.
Significant reduction in the number of inflammatory lesions started right from day 15th of treatment and continued till the end of the study period. Although the mean inflammatory lesion count on day 60 was 1.89 ± 3.06, there were 27 (43.54%) out of 62 subjects who experienced complete relief from inflammatory acne lesions. Also significant relief from all the signs and symptoms of acne vulgaris was observed right from day 15 of the treatment. The results of the current clinical study are in line with the results observed in in vivo and in vitro studies conducted on AHPL/AYCAP/0413 capsule and AHPL/ AYTOP/0213 cream, wherein it was observed that both the drugs possess anti-inflammatory and anti-bacterial activities [3]. Due to anti-bacterial and anti-inflammatory activities, AHPL/ AYCAP/0413 capsule and AHPL/AYTOP/0213 cream are able to provide significant relief from all the symptoms of acne vulgaris.
Almost all the ingredients of AHPL/AYCAP/0413 capsule and AHPL/AYTOP/0213 cream possess anti-inflammatory activity [3-16] Sariva (Hemidesmus indicus) [4] and Manjishtha (Rubia cordifolia) [5] present in the formulations are known to exert anti-inflammatory activity by suppressing the capacity of P. acnes-induced reactive oxygen species (ROS) and pro-inflammatory cytokines which are the mediators of inflammation in acne vulgaris. Acorus calamus is also known to inhibit the production of pro-inflammatory cytokines through multiple mechanisms [5]. Daruharidra (Berberia aristata) [6], Lodhra (Symplocos racemosa) [7], Yashtimadhuka (Glycyrrhiza glabra) [8], Jatiphala (Myristica fragrans) [9], Manjishtha (Rubia cordifolia) [4], Sariva (Hemidesmus indicus) [4,10] Nimba (Azadirachta indica) [11] and Khadira (Acacia catechu) [12] demonstrate antibacterial activity against Propionibacterium acnes, Staphylococcus epidermidis and Staphylococcus aureus, which are known to be responsible for development of acne and various other skin infections. The observed anti-inflammatory and antibacterial activity of AHPL/AYCAP/0413 capsule and AHPL/AYTOP/0213 cream could be the result of synergistic effect of activities of the ingredients present in these formulations.
Significant reduction in non-inflammatory acne lesion count and total lesion count was also observed at the end of the study period. This suggest possible role of AHPL/AYCAP/0413 capsule and AHPL/AYTOP/0213 cream in reducing excessive sebum production. Researchers have reported that, Glycyrrhiza glabra (one of the important ingredients of AHPL/AYCAP/0413 capsule and AHPL/AYTOP/0213 cream) possesses anti-androgenic activity. Glycyrrhiza glabra [13] could have helped in regulating sebum production and thereby reducing non- inflammatory lesions like whiteheads and blackheads.
On the baseline visit, 9.7% cases had acne vulgaris of moderate severity and 90.3% subjects had acne vulgaris of mild severity. At the end of the study, no single case was found to have moderate acne vulgaris. Post inflammatory hyperpigmentation and scarring are common complications of inflammatory acne lesions. Final score of scarring on last follow up visit did not show remarkable difference when compared to baseline suggesting no significant improvement in scarring. Post acne dark spots were counted on every follow up visit. On day 15, 30 and 45 slight increase in this number as compared to baseline was noted but it was not statistically significant. Dark spots appear after resolution of inflammatory acne lesions and it was observed that treatment with AHPL/AYCAP/0413 capsule and AHPL/AYTOP/0213 cream was effective in reducing the number of inflammatory acne lesions. These newly resolving inflammatory acne lesions might have added to the existing number of dark spots leading to increase in the total number of post acne dark spots. Although the number was found to increase on 3rd follow up visits, it was lesser at the end of the study.
The darkness of the index spot was found to have reduced significantly with the treatment. 24.2% subjects reported to have their largest or Index dark spot “completely cured” and 40.3% reported excellent improvement in the darkness of index dark spot at the end of the study. This suggests that AHPL/AYCAP/0413 capsule and AHPL/AYTOP/0213 cream also helped in the resolution of post acne hyperpigmentation. Scaling and itching due to the acne lesions was also found to be reduced significantly at the end of the study. Overall skin colour of the face of the subject assessed using Von Luschan along with Fitzpatrick type scale did not show any significant improvement from the baseline. However it was observed that, subjects initially grouped under darkest skin type category shifted to the lighter skin type category at the end of the study.
As per the global evaluation for overall improvement done as per the physician suggests that 66.2% subjects were reported to have experienced good to excellent improvement, whereas 4.8% subjects reported poor improvement. Feeling of improvement in acne is very much associated with individual’s perception about having clear skin and not just absence of acne. Even if the inflammatory papules, pustules and comedones were resolved significantly in most of the cases, the residual post inflammatory dark spots and scars might have interfered in subject’s ultimate opinion about overall relief from acne.
42 subjects were reported to have at least one adverse event in the course of the 60 days of treatment period. Almost all the adverse events were not related to the study drugs. No post treatment significant change in vital parameters (pulse rate, body temperature, and respiratory rate, systolic and diastolic blood pressure) and body weight was seen in any subject when compared to baseline visit. No post treatment significant change in any laboratory investigation was observed. As per the global evaluation for drug tolerability done by physician and subjects, 98.4% subjects had experienced good to excellent tolerability which indicates significant safety of the study drugs in human subjects.
Almost all the ingredients of AHPL/AYCAP/0413 capsule and AHPL/AYTOP/0213 cream possess anti-inflammatory [4,5,8,14,15] and antimicrobial activity [4,6-11]. Kakamachi (Solanum nigrum) [14,15] and Guduchi (Tinospora cordifolia) [16] in AHPL/AYCAP/0413 capsule are known hepatoprotective herbs that help to improve overall metabolism. They improve liver functions and help in removal of toxins from blood. The ingredients of AHPL/AYTOP/0213 cream also help in reducing hyperpigmentation of the skin. Hence the proven efficacy of these formulations in Acne vulgaris could be attributed to the synergistic action of the various herbs present in the formulations.
It can be concluded that, use of AHPL/AYCAP/0413 capsule and AHPL/AYTOP/0213 cream is safe and significantly effective in reducing inflammatory, non-inflammatory, total lesion counts in acne vulgaris. This treatment is also effective in reducing all the symptoms of acne vulgaris, acne severity as well as post acne dark spots. Thus, study drugs are safe and effective treatment option for acne vulgaris.
Authors are extremely grateful to Mr. Sanjeevan Kanjilal (Managing Director) and Dr. Anisha Kanjilal (Director) of Ari Healthcare Pvt. Ltd., for providing all the research facilities, guidance, courage and moral support. The authors also sincerely thank all the staff of Ari Healthcare Pvt Ltd, Hinjewadi, Pune and clinical research staff of MAM's SS Ayurveda Mahavidyalaya and Sane Guruji Aarogya Kendra, Malwadi, Hadapsar, Pune.
The CTRI registration no. is CTRI/2016/06/007032 (Registered on: 23/06/2016).
All the authors have substantially contributed in the concept, design, literature search and analysis and interpretation of the study data. All the authors have substantially contributed in preparation, editing and review of the manuscript. No author of the study has any conflict of interest.
Citation: Nipankar SU, Deshpande V, Chopade HS (2020) Evaluation of Efficacy and Safety of AHPL/AYCAP/0413 Capsule and AHPL/ AYTOP/0213 Cream in Patients Suffering from Acne Vulgaris. J Clin Exp Dermatol Res. 11:525. DOI: 10.35248/2155-9554.20.11.525
Received: 04-Jun-2020 Accepted: 18-Jun-2020 Published: 25-Jun-2020 , DOI: 10.35248/2155-9554.20.11.525
Copyright: © 2020 Nipankar SU, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.