Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2022)Volume 13, Issue 2
Aim: Treatment of aging is challenging. Topical retinol is one of the actives with more published evidence on its efficacy. The aim of the study was to determine the efficacy and tolerance of a protocol based on the use of high concentrations of retinol (0.5% and 1%).
Materials and methods: A prospective pilot study of clinical experience was conducted in 20 volunteers with some degree of skin aging. They used a treatment protocol during 12 weeks, consisting of a progressively increase concentration of retinol at nights (beginning 0,5% and 1% within the subsequent weeks) combined with a nourishing cream based on growth factors and sunscreen at daytime. Five visits were registered (D0, D15, D50, D70, D90). Objective measurements were carried out (by cutometer, tewameter and Visia) together with the determination of the severity of aging, patient and investigator global assessment (PGA and IGA), improvement of skin aging according to patient and investigator opinion and side effects in semiquantitative scale (0-3).
Results: A significant improvement in skin firmness was obtained from D50, reaching its maximum increase (20%) at D90. This improvement agreed with researcher and patient determinations and with the improvement obtained in D90 on the RAO Goldman aging scale. Regarding tolerance, the increase in transepidermal water loss was, as expected, higher between visits at the beginning of the study. Side effects were higher in D50 when retinol was used more frequently, but decreased subsequently. In most of the patients the side effects were null or slight in all visits.
Conclusion: This specific regimen of retinization consisting of the progressive use of high concentrations of retinol (0.5% and 1%), showed efficacy in improving the firmness and visible signs of skin aging. Furthermore, it was very well tolerated by most of the patients.
Retinol; Efficacy; Tolerance; High concentration; Retinization; Cosmetic; Skin aging
Aging is a complex process influenced by factors inter-related, such as internal factors (chronoaging caused by the accumulation of reactive oxygen species, alterations in DNA, hormonal changes, etc.) and external factors (photoaging caused by solar radiation, pollution, stress, diet, life habit). Signs of skin aging include atrophic skin, xerosis, wrinkles, laxity, dyschromia, increased benign tumors, telangiectasias, skin roughness, and precancerous and cancerous lesions [1-5]. There is increasing knowledge and understanding of different mechanisms by which external aggressors modify our skin and make it age.
Given that no treatment is 100% effective, the current trend is to use combined treatments with synergies providing greater increases in the clinical improvement of skin aging [6,7]. The main antiaging goal would be to improve the skin quality to achieve healthy skin. Recently, a qualified advisory board concluded with strong consensus that good skin quality may be defined as “healthy, youthful in appearance (appearing younger than a person’s chronological age), undamaged skin and that skin quality can be described by four emergent perceptual categories (EPCs): skin tone evenness, skin surface evenness, skin firmness, and skin glow”[8]. Specifically, skin tone evenness is determined by pigmentation, erythema and discoloration; skin surface evenness is determined by pore size, crepiness, wrinkles, scars, hair and clarity; skin firmness category is determined by elasticity, tightness and hydration; and skin glow would be synonymous with luminosity and brightness [8]. The skin quality categories can be affected by multiple tissue layers, therefore topical approaches may not be sufficient. Instead, a combination treatment strategy should be necessary as summarized in Table 1 [8].
| Skin aging treatments |
|---|
| Topical cosmetics, cosmeceutics and drugs (see Skin health and beauty pyramid by Dra. Draelos9) |
| Laser: Fractional laser, intense pulsed light, Q-switched and picoseconds laser, vaporization laser |
| Peelings |
| Radiofrequency, microfocused ultrasound with visualization |
| Botulinum toxin |
| Fillers: Hialuronic acid and others (calcium hydroxylapatite (CaHa)) |
| Platelet-rich plasma |
Table 1: Abstract of the most frequently used treatment in skin aging.
In order to achieve healthy skin we can follow the Skin Health and Beauty Pyramid [9]. The approach to antiaging treatment with cosmeceutics involves three pyramid levels: protection and repair at the base; renewal in the middle and activation and regeneration at the top of the pyramid [9]. The skincare products of the base for protection and repair will be: Sun photoprotection, antioxidants and actives to protect and repair DNA Polypodium leucotomus extract (Fernblock®) or protect the microbiome and fight against environmental pollutants (e.g. Deschampsia antartica (Edafence®), prebiotic, probiotic and postbiotic agents, etc). The actives for the middle layer will be retinoids, alpha-hydroxiacids and moisturizers and the ingredients at the top of the pyramid will be peptides, growth factors (e.g. secretion of Cryptomphalus aspersa (SCA®)) and stem cells.
Focusing on the middle layer, one of the main actives to induce skin renewal is retinoids. There are studies that show the benefit of topical retinoids (retinol, or vitamin A, as well as its biologically active derivative: retinoic acid) in the treatment of skin pigmentation (post-inflammatory hyperpigmentation, melasma), pseudofolliculitis of the beard, scars, acne, psoriasis, alterations in keratinization and skin aging [10]. The clinical improvement observed in terms of skin dyschromia, roughness and wrinkles is the result of retinoid activity on cell nucleus and cytoplasm receptors [10]. Through direct mechanisms retinoids normalize melanocyte activity (decrease melanin transfer) and increase keratinocyte turnover, improving dyschromia and roughness associated with photoaging. Retinol is converted to retinoic acid in a 2-stage oxidation process and is less potent than pure retinoic acid. In addition, through indirect mechanisms retinoids regulate the thickness of the stratum corneum and thereby increase the skin penetration of other substances. Although there is no exact conversion, it is accepted that retinol is 10 times less effective than pure retinoic acid [11], this means that although retinol needs higher concentrations than retinoic acid, in the end it will induce the same histological changes (epidermal hyperplasia and dermal Extracellular Matrix (ECM) component production) as retinoic acid, but without causing retinoids irritation, the most common side effect of retinoids [12]. As mentioned, there is evidence that topical retinol can also stimulate neocolagenesis through indirect mechanisms, improving wrinkles, sagging and elasticity [12-14]. Specifically, it has been demonstrated that topical retinol also stimulates TGF-β/CTGF pathway, the main regulator of ECM homeostasis, and thus improves ECM in aged human skin in vivo [12].
Although retinol appears to be a viable candidate for the treatment of photoaged skin, retinol is easily degraded to biologically inactive forms when exposed to light and air [14,15]. It is important to use stabilized retinol, which is achieved with the use of specific carriers and vehicles. For this reason, the efficacy of retinol in the treatment of photodamage depends greatly on formulation and its mode of delivery to the target area. These two factors will be key determining efficacy and tolerance of the various retinoids present in the market. The development of drug delivery systems may provide improved solubilization, prolonged circulation, reduced toxicity, sustained release and improved efficacy [15]. RetinSphere® technology combine hydroxipinacolone retinoate with retinol in Microsponge® delivery system, which is a new delivery technology that provides sustained release for reducing irritation and enhancing stability ensuring long term efficacy. Microsponge® delivery systems are uniform, spherical polymer particles. Their high degree of cross-linking results in particles that are insoluble, inert and of sufficient strength to adsorb a high degree of actives into the particle and on to its surface. Its large capacity for active entrapment carrying up to three times its weight differentiates it from other types of dermatological delivery systems.
Since one of the main adverse effects of retinoids is irritant dermatitis, it is especially useful to identify a treatment regimen with high concentration retinoids but to ensure optimal tolerability, as is the case with Microsponge® and the combination of anti-inflammatory actives present in the product used in this study, so that the patients can achieve good therapeutic compliance through home care protocols [10,16].
Objective
The aim of the study was to determine the efficacy and tolerance of a protocol based on the use of different concentrations of retinol (0.5% and 1%). The products containing the retinol were applied at night according to which entailed increasing frequently applications of increasingly greater retinol concentrations.
A prospective pilot study of clinical experience was conducted. Twenty volunteers with a diagnosis of facial skin photo aging were included. All study volunteers met the following inclusion criteria: age between 35-65, no treatment in previous 3 months and no desire for pregnancy in the following 3 months, no concomitant diseases, no administration of another topical or concomitant systemic product. The subjects discontinued use of their habitual cosmetics a week before the start of the study. All understood and signed the informed consent. The study was approved by the corresponding ethical committee.
Product under study
The products used in the study were: retinol 0.5% cream, retinol 1% cream, SCA based cream (moisturization), Heliocare Age Active Fluid (photoprotection). All the products used in the study are considered safe and categorised as cosmetic products; their ingredients have an INCI nomenclature.
Protocol
The duration of the study was 12 weeks (90 days). The retinoid use protocol, applied at night, is shown in the Table 2. In addition to the use of the retinoid at night, throughout the study the volunteers applied the transition moisturizer day and night, accompanied by SPF 50 sun protection in the morning application. 5 visits were planned (D0, D15, D50, D70 and D90) and the following evaluations were carried out:
| Visits | D0 basal | D15 | D50 | D70 | D90 |
|---|---|---|---|---|---|
| Treatment protocol | Weeks 1, 2 | Weeks 3, 4 | Weeks 5, 6, 7 | Weeks 8, 9, 10 | Weeks 11, 12 |
| Begining with retinization process: Retinol 0,5% two days and transition cream five days. | Retinol 0,5% four days and transition cream three days. | Retinol 0,5% five days and transition cream two days. | Retinol 1% two days and transition cream five days. | Retinol 1% three days and transition cream four days. |
Table 2: Treatment protocol. Description of the night protocol.
• Clinical assessment of the severity of photoaging according to the RAO Goldman Scale (RGWS): 5-point scale: (1) no wrinkles, (2) visible but fine wrinkles, (3) moderately deep wrinkles, (4) deep wrinkles with defined edges and (5) very deep wrinkles with defined furrows. (D0 and D90).
• VISIA photography (D0, D50, D70 and D90).
• Tewameter for transepidermal water loss measurement. (TM- 300 Microcaya (g/m2/h)) and Cutometer for elasticity and firmness registration. (Cutometer Dual MPA 580). (D0, D50, D70 and D90)
• Severity of photoaging as perceived by the Patient (PGA) and Investigator (IGA): 0: none, 1: mild, 2: moderate, 3: intense. (D0, D15, D50, D70, D90).
• Side effects: peeling (0-3), erythema (0-3), burning (0-3), tightness (0-3), others. Being 0: null, 1: mild, 2: moderate, 3: intense. (D15, D50, D70, D90).
The cosmetic treatment would be considered effective if it improves the measurements associated with photoaging (cutometer, severity of aging RAO-Goldman scale, severity of aging perceived by patient and researcher Physician's Global Assessment (PGA) and Investigator’s Global Assessment (IGA)). Treatment will be considered well tolerated if changes in Trans Epidermal Water Loss (TEWL) are transient and if patients do not report significant adverse effects.
Statistical study
The non-parametric Wilcoxon text at each of the evaluation times will be used for ordinal efficacy variables: Clinical assessment of degree of aging perceived by patient and researcher (RGWS), degree of desquamation and erythema, degree of improvement perceived by patient, degree of improvement perceived by investigator and intensity of side effects.
In the case of the quantitative variables of skin hydration, the Student's T-test will be used for paired variables or the Wilcoxon test, depending on whether or not these variables meet normality. The most common centralization, dispersion and position values will be used as descriptors in all cases: Mean, standard deviation and 25th, 50th and 75th percentiles. A difference with respect to baseline will be considered significant, when the level of significance obtained is less than 5% (p≤0.05). The Statistical Package for the Social Sciences (SPSS) V24.0 software will be used to carry out the statistical calculations.
Efficacy
The results obtained regarding the efficacy of the treatment regimen were
Skin firmness (cutometer®): After 50 days of continuous product use, skin firmness improved an average of 13% with respect to baseline. This difference was statistically significant (p<0,05). The higher increase in skin firmness (an average of 20% with respect to baseline) was reached after 90 days of continuous product use. The difference was statistically significant (p<0.05), (Figure 1). Most of the volunteers presented significant improvement in skin firmness starting at D50. At the end of the study, 83% of the population studied showed significant firmness improvement (p<0.05). (Figure 2).
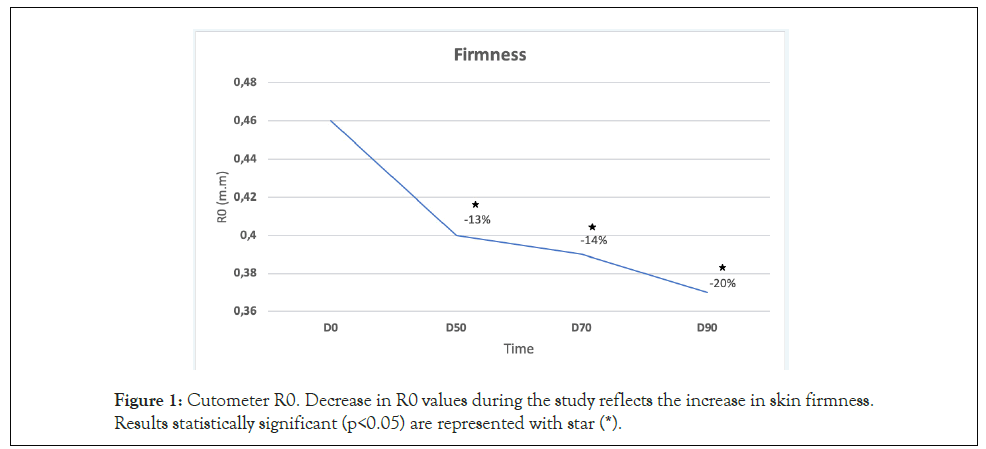
Figure 1: Cutometer R0. Decrease in R0 values during the study reflects the increase in skin firmness. Results statistically significant (p<0.05) are represented with star (*).
Figure 2: Percentage of volunteers with improvement in skin firmness. Most volunteers presented significant improvement in skin firmness starting at D50. Note: (*) Indicates statistically significant results (p<0.05).
Cutaneous elasticity: No significant changes were observed with respect to elasticity (R2, cutometer®).
Rao-Goldman aging scale: at the end of the study (D90) a significant improvement in the score according to this scale was observed.
IGA: After 90 days of continuous product use, significant differences were observed with respect to D0, p <0.05. Before applying the product, the investigator classified 70% of the volunteers presenting as moderate aging. 90 days after application, this percentage decreased to 50% (Figure 3).
Figure 3: Perception of aging by the investigator (IGA). Significant improvement shown in D90. 
PGA: After 90 days of continuous product use, significant differences were observed with respect to D0, p <0.05. At the beginning of the study, before applying the product, 60% of the volunteers rated their aging as moderate or intense. At 90 days after applying the product, this percentage dropped to 40% moderate aging and 0% intense (Figure 4).
Figure 4: Perception of aging by patient (PGA). Significant improvement in D90. 

Investigator perception of improvement: Significant improvement perceived by the investigator was obtained at all times starting from D50. After 50 days of treatment with the 0.5% concentration, the investigator observed a significant improvement, with a mild to moderate improvement observed in 80% of the patients, p<0.05. After 90 days of treatment, 100% of the patients showed some degree of improvement, of which 60% were marked improvement (Figure 5).
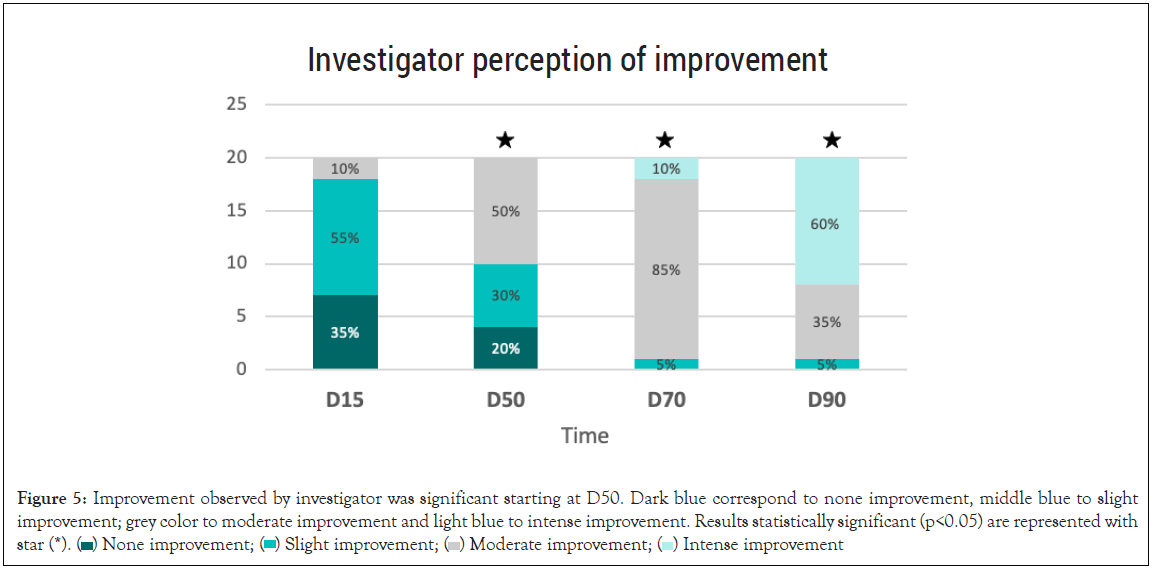
Figure 5: Improvement referred by researcher was significant at D50. Dark blue correspond to none improvement, middle blue to slight
improvement; grey color to moderate improvement and light blue to intense improvement. Results statistically significant (p<0.05) are represented with
star (*). 
Patient perception of improvement: A significant improvement perceived by the patient was observed after 70 and 90 days of treatment. 95% of the volunteers reported moderate to intense improvement (p<0.05) (Figure 6).
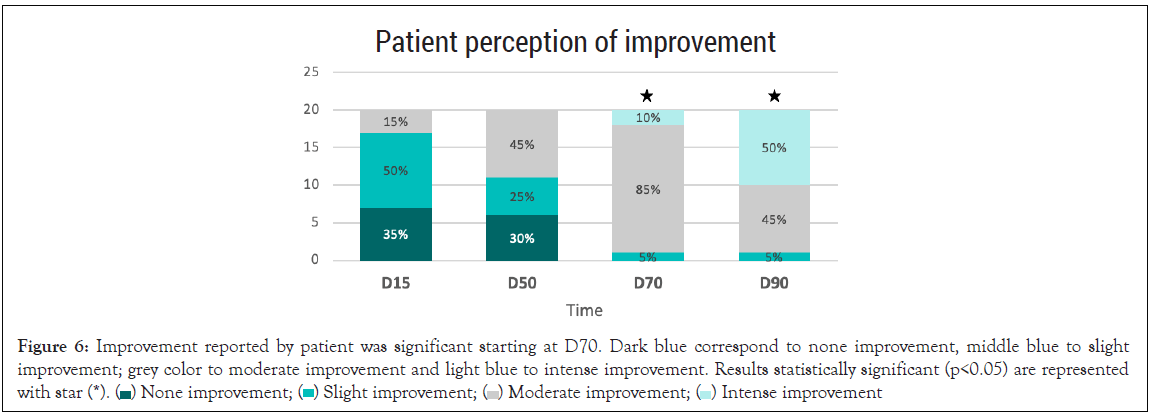
Figure 6: Improvement referred by patient was significant at D70. Dark blue correspond to none improvement, middle blue to slight
improvement; grey color to moderate improvement and light blue to intense improvement. Results statistically significant (p<0.05) are represented with star (*). 
Tolerance
There were no dropouts, so all the recruited volunteers completed the study. It should be noted that of the 20 volunteers, 3 of them (P3, P4 and P20) had a skin type with mild rosacea at the time beginning of the study.
Skin barrier function (TEWL): After 50 days, 70 days and 90 days of continuous product use, TEWL levels were significantly higher compared to the baseline visit. With respect to basal values, ,as we expected, the increase was greater at the beginning of the treatment: in D50 we observed an increase in TEWL of 32%, while at D70 it remained practically stable with respect to D50 (31% compared to baseline). Finally, at D90 TEWL increased by 13% compared to D70 (45% compared to baseline). Therefore, the greatest increase obtained between consecutive visits was obtained between D0 and D50.
Side effects: After 50 days, 70 days and 90 days of continuous product use, a significant difference (p<0.05) in peeling severity was observed with respect to D15, and as well as for erythema and tightness at D50 (p<0.05). Most of the volunteers did not have peeling, erythema or tightness or if they did it was mild (Figures 7-9). No significant differences were observed in terms of the burning sensation in the successive visits with respect to D15. Most of the volunteers described it as null or mild at all visits. Only 15% described it as intense at D50, which later in the following visits that percentage dropped to 5 and 10% at visits D70 and D90 (Figure 10).
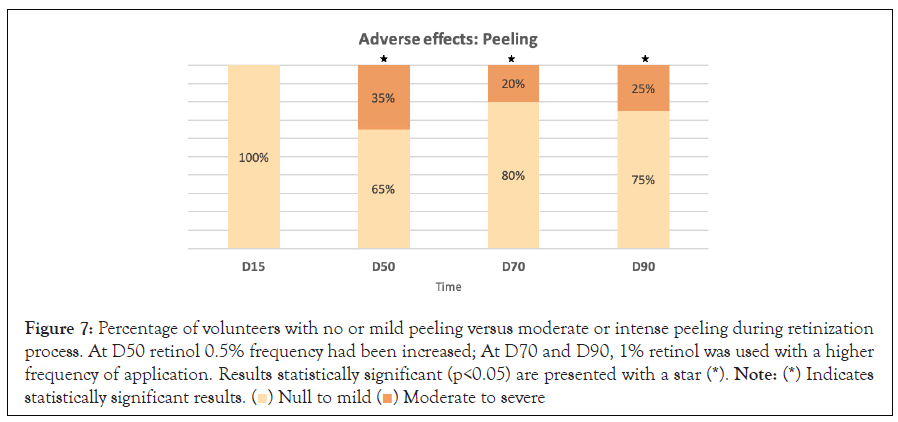
Figure 7: Percentage of volunteers with no or mild peeling versus moderate or intense peeling during retinization
process. At D50 retinol 0.5% frequency had been increased; At D70 and D90, 1% retinol was used with a higher
frequency of application. Results statistically significant (p<0.05) are presented with a star (*). Note: (*) Indicates
statistically significant results. 
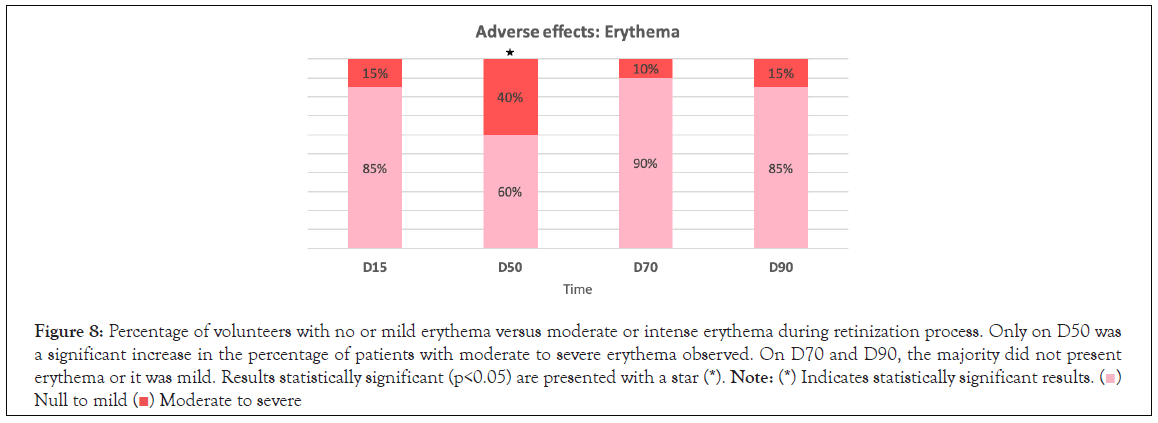
Figure 8:Percentage of volunteers with no or mild erythema versus moderate or intense erythema during retinization process. Only on D50 was
a significant increase in the percentage of patients with moderate to severe erythema observed. On D70 and D90, the majority did not present
erythema or it was mild. Results statistically significant (p<0.05) are presented with a star (*). Note: (*) Indicates statistically significant results.
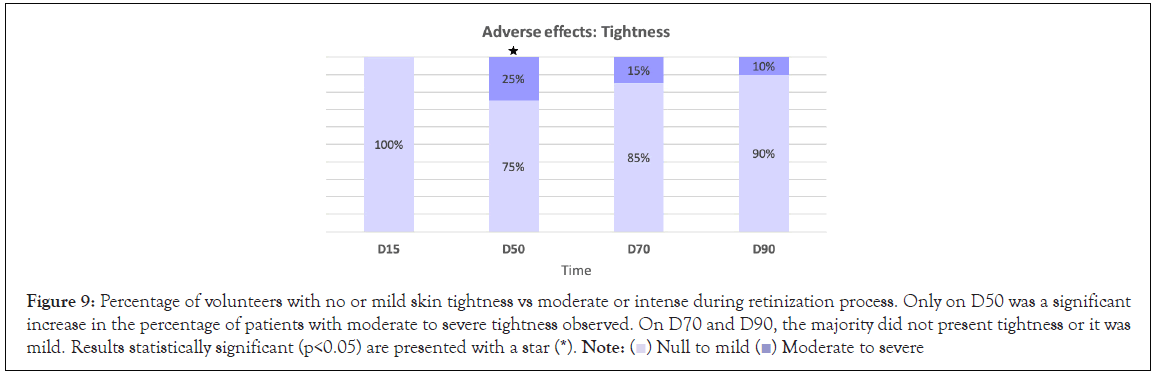
Figure 9: Percentage of volunteers with no or mild skin tightness vs moderate or intense during retinization process. Only on D50 was a significant
increase in the percentage of patients with moderate to severe tightness observed. On D70 and D90, the majority did not present tightness or it was
mild. Results statistically significant (p<0.05) are presented with a star (*).
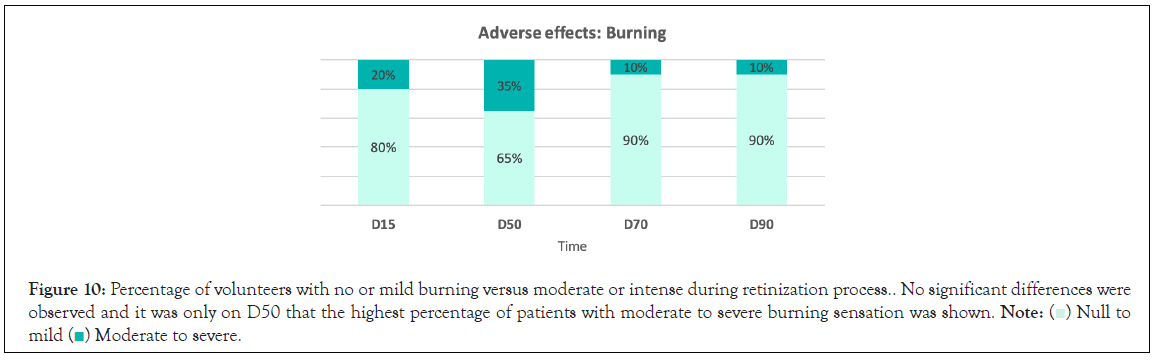
Figure 10: Percentage of volunteers with no or mild burning versus moderate or intense during retinization process.. No significant differences were
observed and it was only on D50 that the highest percentage of patients with moderate to severe burning sensation was shown.
Following images of some volunteers reflect the improvement in skin surface texture, tone and glow. (Figures 11-14).
Figure 11: Notable improvement in skin texture and unification of skin tone, evident from the first control.
Figure 12: Notable improvement in skin texture and unification of tone between D0 and D50 and D90.
Figure 13: Notable improvement between D0 and D50 in luminosity and periocular wrinkles in a patient with mild telangiectatic rosacea, which increases by D7. The improvement in wrinkles and luminosity continues at D90. A slight increase in erythema was observed with the higher concentrations of retinol.
Figure 14: Notable improvement compared to baseline in this volunteer with rosacea who presented more redness at D50 vs D0 and D70 and improved at D90.
Topical retinoids have been shown to be effective and safe in treating most signs of skin aging [17], due in part to their ability to normalize epidermal structure and organization, thin of the corneal layer, inhibit melanin synthesis, and induce the synthesis of collagen and elastin fibers [18].
Of all the retinoids, tretinoin (all-trans-retinoic acid, first generation retinoid) is the most frequently studied in photoaging. Limitation to its use are due to its problems with tolerability, as development of irritative dermatitis in the areas of application has been described in patients with skin sensitive to retinoids. Tolerance can however improve by using other less irritating retinoids such as retinaldehyde and retinol. Specifically, retinol is a retinoid whose anti-aging effect was first described in 1995 [19]. Topically applied retinol has been shown to induce skin improvement at the histological and molecular level (20). It induces epidermal thickening and increases the expression of Cellular retinoic acid-binding protein (CRABP) II and CRABP mRNAs. I, II and proteins, with fewer side effects than tretinoin [20,21]. In addition, it inhibits the activation of Matrix Metallo Proteinases (MMPs) induced by Ultra Violet (UV) rays and stimulates collagen synthesis in photoaged skin [18]. It is a very unstable molecule and thus its correct delivery, conservation and presentation is essential [22]. Previously published evidence showed that retinol serums (0.25%, 0.5%, 1.0%) were safe and effective with equivalent/or better performance and tolerability than tretinoin creams [23].
Given the high efficacy and better tolerance of retinol compared to tretinoin, we suggest that a good anti-aging treatment protocol would be one that combines a powerful retinol regimen with good tolerance. For this reason, a retinization regimen was proposed with a progressive increase in the retinol concentrations of different formulations in combination with a transitional nourishing cream, with the aim of increasing tolerance without losing effectiveness. The results obtained in our study confirmed that this treatment regimen was effective given the significant improvement in skin firmness and in the patient and investigator perceptions of severity of skin aging. The improvement in skin firmness was obtained from D50, which could be attributed to the dermal action of retinol on fibroblasts, whose stimulation and synthesis of new collagen fibers takes several weeks, usually around two months. The improvement in skin firmness continued to increase over the next visits, suggesting that continuous use of the protocol perpetuates retinol dermal stimulation.
Significant improvement on the Rao-Goldman severity aging scale was obtained by reaching a mild to moderate grade at the end of the study in 80% of the volunteers compared to 65% who showed mild to moderate grade at the beginning of the study. In addition, the investigator and patient perception of improvement was significant from D50 and D70, respectively, reaching some degree of improvement at the end of the study in 100% of the volunteers. It should be noted that 60% of patients showed intense improvement. In parallel, 83% of volunteers showed a significant improvement of skin firmeness at the end of the study. These results therefore reflect the great satisfaction of the participating volunteers.
Regarding the side effects caused by this retinol regimen, as could be expected, the barrier function was altered, as demonstrated by an increase in TEWL, but it should be noted that the increase between visits was more marked in the first phases of retinization with the greatest increase occurring at D50 when retinol 0,5% was applied five nights per week; on the following visits however most of the volunteers referred side effects as none or light, even with higher retinol concentration (1%). This can be explained by two factors: the skin gets used to higher concentrations of retinoids when a correct retinization protocol is used and on the other hand, the retinol action on keratinocyte turnover and horny layer regulation leads to a healthier skin and stronger epidermal protection activity after several weeks of retinol use. [24].
As with TEWL, some of the side effects increased significantly at D50, when 0,5% retinol was being applied more frequently, reaching moderate or intense intensity in a large percentage of patients. However, during successive visits the majority returned to have no or mild intensity of side effects even with the higher retinol concentration (1%). This indicates the importance of carrying out an appropriate retinization protocol to obtain greater tolerance to high concentrations of retinoids. Peeling was also significantly higher also at D50, reaching moderate or intense grade in 35% of patients. In this case, on subsequent visits it was the side effect that decreased the least, but even so, most of the patients did not peel and of those who did, the majority were mild or moderate peeling at all visits. Peeling is a visible sign of epidermal renewal and the reorganization of keratinocytes, which clinically usually manifests itself with improvement in brightness, dark and greater uniformity of tone [19,20].
Based on side effects, the use of 0.5% retinol was very well tolerated. When patients used it more frequently (D50) the side effects increased temporarily, although the severity of the side effect was classified as mild or moderate in most of the participants. Subsequently, the side effects tended to decrease even with higher retinol concentration (1%), as the skin adapted and tolerated the retinol use and as the retinol induces structural and functional skin improvements [23]. In no case did it cause the suspension of treatment or withdrawal from the study by participants.
With the proposed retinization regimen, it was observed that when 0.5% retinol was used more frequently, the side effects increased temporarily but decreased in the following weeks, even with the use of a higher concentration of retinol (1%), reaching an excellent tolerance in most cases. This shows that the retinization process is useful in increasing the tolerability of high retinol concentrations.
With respect to efficacy, a significant improvement in skin firmness was observed starting at D50, reaching its maximum increase of 20% at D90. This improvement was in accordance with the improvement observed by both investigator and patients and with the improvement obtained at D90 on the RAO Goldman skin aging scale. This retinol regimen is thus effective and safe for the management of skin aging.
This work was supported by grants from Cantabria Labs.
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
[Crossref] [Google schloar] [Pubmed]
Citation: Truchuelo MT, Rodríguez PU, Maria V (2022) Evaluation of Effectiveness and Tolerance of a Cosmeceutic Regimen Based on the Topical Retinoids. J Clin Exp Dermatol Res. 13:606.
Received: 23-Mar-2022, Manuscript No. JCEDR-22-16379; Editor assigned: 28-Mar-2022, Pre QC No. JCEDR-22-16379 (PQ); Reviewed: 11-Apr-2022, QC No. JCEDR-22-16379; Revised: 18-Apr-2022, Manuscript No. JCEDR-22-16379(R); Published: 25-Apr-2022 , DOI: 10.35248/2155- 9554.22.13.605
Copyright: © 2022 Truchuelo MT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.