Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research - (2023)Volume 13, Issue 6
Water is a universal solvent on earth and sources such as wells, rivers, springs, boreholes, and other freshwater bodies typically serve as a pathway for contaminants to enter the ecosystem while also supplying water for domestic and drinking purposes. The activity concentrations of radionuclides in borehole water in Igbajo town were assessed using gamma-ray spectroscopy, so as to effectively determine the degree of radiological risk to the environment and its inhabitants, the outcomes were used to calculate all the radiological impact parameters. The activity concentrations obtained for 40k, 238U and 232Th ranged from 7.25 ± 0.60 to 62.15 ± 4.48 Bq.L–1; 3.08 ± 0.45 to 15.24 ± 3.07 Bq.L–1 and 1.08 ± 1.10 to 17.75 ± 1.59 Bq.L–1 and with average values of 23.12 ± 1.59 Bq.L–1, 6.27 ± 2.01 Bq.L–1 and 7.01 ± 0.89 Bq.L–1 respectively. The Annual Effective Dose (AED) for ingested radionuclide in drinking water for an adult was 1.4872 μSv.yr–1. The Radium Equivalent Activity Index, Raeq obtained was 34.9186 Bq.L–1. The estimated hazard indices Hint and Hext were 0.04876 and 0.06574 respectively. The Excess Lifetime Cancer Risk, ELCR (× 10–6) was 5205.15. With a lifetime expectancy of 70 years, this high value suggests that there is a high chance of developing cancer. The value of the Annual Gonadal Equivalent Dose (AGED) in the water was 55.9175 μSv.yr–1. The estimated Gamma Index, Iγ was 0.1273 mSv.yr–1. There is a significant health hazard to the environment and people living in the area owing to the radioactivity contents and radiological impact parameters.
Borehole waters; Radiation hazard parameters; Toxicity; Cancer risk
The contamination and pollution of nature are results of human activity. These ongoing activities have severely deteriorated the natural ecosystem and caused it to be vulnerable to natural radiation from the earth and space [1].
Due to the fact that borehole water quality differs from source to source, examination of the suitability of this water is important in Nigeria where drinking of borehole water has significantly increased. Different analytical techniques have been used in different nations to quantify the radioactivity levels in drinking water [2]. Naturally occurring 40k, 232Th and 238U series are the sources of radioactivity in geological materials, primarily soil and rocks. [3].
Radiation levels are higher in igneous rocks (granite), and lower in sedimentary rocks. However, rare exceptions abounds, including phosphate and shales rocks that have comparatively high radioactive concentrations [4]. Depending on the dose ingested, radiation has consequences on people. High radiation doses have the potential to change human DNA, although low doses may not have any discernible effects. Both random and predictable biological effects of radiation exposure exist [5]. A deterministic effect takes a dose threshold, and the severity of the effect is dose- related, as in the case of skin reddening, but stochastic effects do not require a dose threshold and are determined by the molecular mechanisms at play, as in the case of cancer or a hereditary defect.
NORM (Naturally Occurring Radioactive Material) refers to radioactive elements with a long half-life, such as uranium, thorium, and potassium, as well as any of their decay products, including radium and radon. These elements existed in the Earth’s crust and atmosphere but are concentrated in particular places, such as deposits of extractable uranium ore. Industrial with NORM related issues include: Oil and gas production; coal mining and combustion; mineral sands (rare earth minerals, titanium, and zirconium); metal mining and smelting; fertilizer (phosphate); recycling; building; and uranium mining and all related fuel cycle activities [6].
Uranium series disequilibrium techniques were first applied by Rosholt et al. [7], and subsequently by Titayeva et al. [8]. Two facts form the basis of these applications. Firstly, 234U and 238U are often under secular equilibrium in rocks. Second, natural levels of 238U differs within a very wide range (101 to 103 mBq/l) [9]. These elements work together to provide the foundation of a very effective geochemical instrument. Some daughter radionuclides exhibit preferential leaching and this can result from a variety of physical effects. For duration comparable to their individual half- lives, the initial disequilibrium of other long-lived radionuclides continues. According to geochemical theory, radium and uranium are soluble species, with natural activity concentrations typically higher than 1 mBq.L–1 [10].
The purpose of this study is to assess the radioactivity and evaluate the risk parameters for the samples of water collected from Igbajo town, to ascertain the level to which the inhabitants are disposed to to radiation hazards.
The study area
It is situated in Igbajo town, Boluwaduro Local Government, Osun State, South-west Nigeria on latitude 7°54’24” N and longitude 4°48’44” E.
The community comprises range of mountains and adjacent basins and its environs are strategically defensible due to surrounding rocky topography with stalwart outcrops. The major tectonic actions range to hornblende-granite-biotite, muscovite- granite-tourmaline-gneiss, gneiss, biotite-gneiss-granite, variably migmatized gneisses and pegmatite intrusions. The dominant rock is quartz schist and quartz and variably biotite-garnet-schist gneiss and biotite-garnet-schist [11].
Sampling
A collection protocol was established and was strictly adhered to. These include a collection procedure, using suitable bottles and employing appropriate methods for preservation so as to minimize the influence of adsorption.
A total of six (6) samples were collected each with 2 L-sized plastic bottles, which was washed and rinsed with dilute Hydrochloric acid (0.1 M HCl). The samples collected were acidified with 1 M hydrochloric acid to attain a pH<2 so as to prevent adsorption of the radionuclide on the walls of the container [12]. The samples were then transported to the laboratory for further processing prior to instrumental analysis.
Measurement and analysis
The procedure involved using a thallium activated Canberra vertical high purity 3 × 3 Sodium Iodide [NaI(Tl)] detector connected to ORTEC 456 amplifier. The detector was protected by about 15 cm thick lead on the four sides and 10 cm thick at the top. About 2.0 KeV resolution and 33% efficiency at 1.33 MeV was accomplished in the system with 27000 s counting time. For calibration, the usual sources recommended by the International Atomic Energy Agency (IAEA) were employed [13]. From the counting spectra, the activity concentrations of 40k, 238U and 232Th were determined using computer program. Various peaks were taken into account. Prior to arriving at the calculation of each activity levels, in comparison to 1460 KeV (40k) for 40k, 1764.5 KeV (Bi-214) for 238U, and 2614.5 KeV (Ti-208) for 232Th.
The activity concentration (C) of the radionuclide can be evaluated after subtracting decay correction with [14];
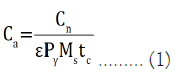
Where, Ca=Activity concentration of radionuclide (Bq.L–1)
Cn=Net counts of radionuclide in the samples
ε=Absolute counting efficiency of the detector system
Pγ=Gamma ray emission probability (gamma ray yield)
Ms=The mass of the sample (kg)
tc=Total counting time
Radiological hazard Parameters
Absorbed dose rate (D): The rate, D (nGy/h) with respect to activity concentration of 238U, 232Th and 40k is calculated using equation 2 [15];
D=0.462CU+0.604CTH+0.0417Ck ……… (2)
Annual Effective Dose (AED): The annual effective dose due to 40k, 238U, and 232Th ingestion was calculated using equation 3 [16,17].
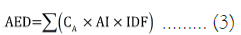
Where, AED=Annual Effective Dose (mSvyr–1),
CA=Activity concentration of the radionuclides (BqL–1)
AI=Average person’s intake of water per year (730 Lyr–1 for adult)
IDF=Ingestion Conversion Factors (ingestion dose coefficient);
IDF for adults for 40k, 238U, and 232Th is 6.2 × 10-9, 2.3 × 10-7, and 4.5 × 10-8 Sv.Bq–1 for respectively.
Radium Equivalent Activity Index (Raeq): It represents the weighted sum of activities concentration of 40k, 238U and 232Th. It is usually calculated to estimate the radiological risks related with the three radionuclides. It is assumed that 1 Bq.Kg–1 of 238U, 0.7 Bq.Kg–1 of 232Th and 13 Bq.Kg–1 of 40k produce the same gamma- ray dose. It can be defined empirically using equation 4 [18] as:
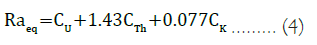
Where, CU, CTh and CK are the radioactivity concentration of 40k, 238U and 232Th respectively.
Excess Lifetime Cancer Risk (ELCR): The ELCR was assessed using equation 5 below.
ELCR=AED × DL × RF ……… (5)
Where, AED=Annual Effective Dose
DL=Average Duration of Life (70 years)
RF=Risk Factor, for stochastic effects, ICRP uses 0.05 for public
Radiation hazard indices: Both the external radiation hazard index (Hext) and the internal radiation hazard index (Hint) were estimated using equation 6a and 6b [14]:
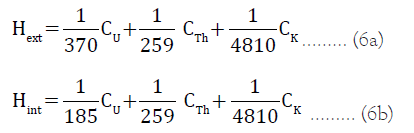
Where CU, CTh and CK are the radioactivity concentrations of 40k, 238U and 232Th respectively.
The values of both the Hext and Hint should be below unity for the radiation risk to be negligible. Internal exposure to radon is very dangerous thus lead to respiratory diseases like lung cancer, asthma etc.
Gamma index (Iγ): It is used for the estimation of gamma radiation hazard related with the natural radionuclide in specific samples. It can be calculated using equation 7:
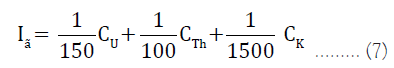
Iγ ≤ 1
Where CU, CTh and CK are the radioactivity concentrations of 238U, 232Th and 40k in water samples.
An increase in Iγ greater than 1 often results to radiation risk which may result to the modification of human cells thereby causing cancer.
Value of Iγ=1 corresponds to an annual effective dose of less than or equal to 1 mSv.
Value of Iγ=0.5 corresponds to annual effective dose less or equal to 0.3 mSv [14].
Annual Gonadal Equivalent Dose (AGED): The gonads, bone cells and bone marrow are centre of interest by UNSCEAR (2000) because of their sensitivity to radiation. As the AGED increases, the bone marrows are affected, causing damage of the red blood cells and are then substituted with white blood cells. This results in a blood cancer known as leukemia.
AGED can be evaluated using equation 8:
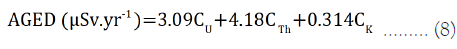
Where CU, CTh and CK are the radioactivity concentration of 238U, 232Th and 40k in water samples.
Chemical toxicity risk
The chemical toxicity risk was calculated using the Lifetime Average Daily Dose (LADD) of uranium through drinking water intake, and related it with the Reference Dose (RFD) of 0.6 µg.kg–1.day–1 [19] produced a hazard quotient using as standards for uranium in several foreign organizations.
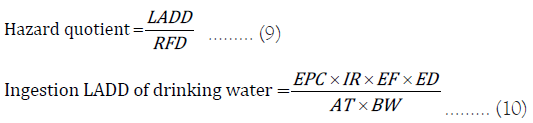
Where, LADD=Lifetime Average Daily Dose (μg.kg–1.day–1)
EPC=Exposure Point Concentration (μg.L–1); IR=Water Ingestion Rate (L.day–1)
EF=Exposure Frequency (days.year–1); ED=Total Exposure Duration (years)
AT=Average Time (days); BW=Body Weight (kg)
Using IR=2 L.day–1; EF=350 days; ED=45.5 years; AT=16,607.5 (from 45.5 × 365); BW=70 kg (standard man)
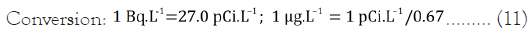
Activity concentrations of radionuclides in the samples
The radionuclides activity concentrations in the water samples taken from the study areas are presented in Table 1 below. The activity concentration of 40k in the borehole water samples ranged from 7.25 ± 0.60 to 62.15 ± 4.48 Bq.L–1 with an average value of 23.12 ± 1.59 Bq.L–1 and that of 238U ranged between 3.08 ± 0.45 and 15.24 ± 3.07 Bq.L–1 with an average value of 6.27 ± 2.01 Bq.L–1 while that of 232Th in the samples ranged from 1.08 ± 1.10 to 17.75 ± 1.59 Bq.L–1 with an average of 7.01 ± 0.89 Bq.L–1.
| Water Samples | 40k (Bq.L–1) | 238U (Bq.L–1) | 232Th (Bq.L–1) |
|---|---|---|---|
| BAB | 9.92 ± 0.61 | 4.52 ± 1.89 | 1.79 ± 0.13 |
| BAP | 13.44 ± 1.05 | 6.66 ± 1.37 | 5.92 ± 0.51 |
| COM | 29.70 ± 1.81 | 3.09 ± 1.03 | 1.17 ± 0.75 |
| ESW | 16.24 ± 0.98 | 5.01 ± 4.23 | 1.08 ± 1.10 |
| ISA | 62.15 ± 4.48 | 15.24 ± 3.07 | 14.34 ± 1.26 |
| ODF | 7.25 ± 0.60 | 3.08 ± 0.45 | 17.75 ± 1.59 |
| MEAN ± S.D. | 23.12 ± 1.59 | 6.27 ± 2.01 | 7.01 ± 0.89 |
| WHO | 10.00 | 10.00 | 1.00 |
Note: BAB: Babalaje; BAP: Baptist; COM: Community; ESW: Essawe; ISA: Isao; ODF: Odofin; WHO: World Health Organization; S.D: Standard Deviation
Table 1: Activity concentration of 40k, 238U and 232Th in the borehole water samples.
Hence, ODF sample had the lowest concentration of 40k while ISA had the highest concentration. The highest and lowest activity concentration values of 238U were found in ISA and ODF respectively. While the highest concentration of 232Th was found in ODF, the lowest was found in ESW. These variations are attributed to the different sources of water samples. Thus, 40k contributed the largest activity concentration while 238U contributed the least activity in the samples.
Researcher’s evaluation of potential radiological hazards to humans is made possible by knowledge regarding the distribution of these radionuclide activities found in natural materials. However, they will be utilized to calculate all radiological impact hazard parameters so that we can know to what extent the local population are exposed.
Radiological hazard parameter in water-absorbed dose rate (D)
The absorbed dose rate (nGy.h-1) in the samples were evaluated with equation (2), and the results presented in Table 2. The absorbed dose rate values ranged from 3.3727 to 18.2939 nGy.h-1 with an average value of 8.0922 nGy.h-1 for the study areas. From this study, the estimated average value was lesser than the world average value of 57 nGy.h-1 [3], and hence, poses no severe health risk.
| Sample Code |
D (nGy.h–1) |
Hint | Hext | Raeq (Bq.Kg–1) |
Iγ (mSv.yr–1) |
AGED (μSv.yr–1) |
AED (μSv.yr–1) Adults |
ELCR (× 10-6) |
|---|---|---|---|---|---|---|---|---|
| BAB | 3.5831 | 0.0212 | 0.0334 | 13.2435 | 0.0547 | 24.5639 | 0.4939 | 1728.72 |
| BAP | 7.2130 | 0.0436 | 0.0617 | 22.9405 | 0.1126 | 49.5452 | 1.2735 | 4457.53 |
| COM | 3.3727 | 0.0190 | 0.0274 | 33.6600 | 0.0521 | 23.7645 | 0.4324 | 1513.30 |
| ESW | 3.6441 | 0.0211 | 0.0346 | 19.0349 | 0.0550 | 25.0947 | 0.4194 | 1467.95 |
| ISA | 18.2939 | 0.1094 | 0.1507 | 87.4418 | 0.2864 | 126.5479 | 3.1896 | 11163.64 |
| ODF | 12.4463 | 0.0783 | 0.0867 | 33.1908 | 0.2029 | 85.9887 | 3.1142 | 10899.76 |
| MEAN | 8.0922 | 0.0488 | 0.0657 | 34.9186 | 0.1273 | 55.9175 | 1.4872 | 5205.15 |
| UNSCEAR | 57.00 | 1.00 | 1.00 | 370.00 | 1.00 | 300 | 1000.00 | 290.00 |
Note: BAB: Babalaje; BAP: Baptist; COM: Community; ESW: Essawe; ISA: Isao; ODF: Odofin; UNSCRAE: United Nations Scientific Committee on the Effects of Atomic Radiation; AED: Annual Effective Dose; AGED: Annual Gonadal Equivalent Dose; ELCR: Excess Lifetime Cancer Risk.
Table 2: Radiation hazard parameters for the water samples.
Annual effective dose (μSv.yr-1)
The annual effective dose resulting from the ingestion of water samples were calculated using equation (3). The values ranged from 0.4194 to 3.1896 μSv.yr-1 with an average value of 1.4872 μSv.y-1 for the study areas.
It was eminent that the values assessed for all the water samples were lower than the world average value of 1000 μSv.yr-1 hence it is within the safe limit.
Radium equivalent activity index (Raeq)
The Radium equivalent activity index (Raeq) from water samples were estimated using equation (4).The result ranged between 13.2435 to 87.4418 Bq.Kg-1 with an average value of 34.9186 Bq.Kg-1for the study areas. The obtained average value was below the world average value of 370 Bq.Kg-1 and hence poses no significant health hazard [3].
Excess Lifetime Cancer Risk (ELCR)
The excess life time cancer risk for the analysed water samples were evaluated using equation (5). The ELCR ranged from 1467.95 × 10-6 to 11163.64 × 10-6 with an average of 5205.15 × 10-6. It is worthy of note that the obtained average value was greater than the world average value of 0.2 × 10-3 (200 × 10-6) [3]. This suggests that there may be a significant risk of cancer associated with spending a 70 year average in this environment without associating with other environment for food and shelter.
The high value of the ELCR index for water samples is due to high AED caused by high activity concentration of 40k radionuclide in the samples.
Radiation hazard indices
The radiation hazard indices in water samples, both the external and the internal were evaluated using equation (6a) and (6b) respectively. The external radiation hazard index (Hext) ranged between 0.0274 and 0.1507 with an average of 0.0657 while the internal radiation hazard index (Hint) ranged from 0.0190 to 0.1094 with an average of 0.0488 for the study. The two values were found to be lesser than the world average value of unity, therefore poses no significant health hazard [3].
Gamma index (Iγ)
The gamma indices for the samples were calculated using equation (7). The average value estimated for the water samples was 0.1273 mSv.yr–1 for values ranging between 0.0521 and 0.2864. This value is within the safe limit of less than unity, the universal standard.
Annual Gonadal Equivalent Dose (AGED)
The AGEDs of the water samples were calculated using equation (8). The average value estimated was 55.9175 μSv.yr–1 for values ranging between 23.7645 and 126.5479. This value is within the safe limit of less than the universal standard.
The result presented in Table 3 showed that the exposure dose ranged from 3.40 to 16.83 μg.kg–1.day–1. The LADD value was observed highest in ISA sample. This might be as the result of the depth of the water source and the geochemistry.
| Sample | 238U (Bq.L–1) | 238U (pCi.L–1) | 238U (μg.L–1) | LADD (μg.kg–1.day–1) |
|---|---|---|---|---|
| BAB | 4.52 | 122.04 | 182.16 | 4.99 |
| BAP | 6.66 | 179.82 | 268.40 | 7.35 |
| COM | 3.09 | 83.43 | 124.53 | 3.41 |
| ESW | 5.01 | 135.27 | 201.90 | 5.53 |
| ISA | 15.24 | 411.48 | 614.17 | 16.83 |
| ODF | 3.08 | 83.16 | 124.12 | 3.40 |
Note: BAB: Babalaje; BAP: Baptist; COM: Community; ESW: Essawe; ISA: Isao; ODF: Odofin; LAAD: Lifetime Average Daily Dose
Table 3: The activity concentrations, mass concentrations and estimated Lifetime Average Daily Dose (LADD) of Uranium in the borehole water samples in the study area.
By comparing the Lifetime Average Daily Dose (LADD) obtained in this study and the Reference Dose (RFD) (0.6 µg.kg-1.day- 1), the acceptable level, the chemical toxicity hazard because of the uranium in the water samples were all above the RFD. This suggests that there are health hazards associated with uranium in the water samples which are basically due to the chemical toxicity risk.
The activity concentrations of 40k, 238U and 232Th in borehole water samples in Igbajo town, Boluwaduro local government, Osun State, Nigeria have been examined and the possible radiotoxicity has been documented in this study. The average values of the activity concentrations of 40k, 238U and 232Th exceeded the recommended value by the WHO but a slight difference in the value of 238U.
Similarly, the activity concentrations were related to the mass concentrations of uranium in the samples and these were found to vary from 124.12 μg.L–1 to 614.17 μg.L–1. This indicates that the measured mass concentrations of uranium in the borehole water in the area were relatively high when compared with the recommended safe limits by some various international organizations. It was inferred that the human risk due to uranium content in water may likely be to the chemical toxicity of uranium as a heavy metal, however, this study represents a valuable radiometric data that are vital tools in radio-epidemiological assessment, diagnosis and prognosis of radionuclide-induced diseases to the population of the studied area.
Recommendations
• Effective management of mining and milling activities should be practiced
• Promotion of organic fertilizer (green chemistry is safer and cheaper) instead of synthetic ones.
• Treatment of water should be encouraged and practiced
• Adequate socialization on the risk associated with radionuclides and ways to remediate it in the society should be encouraged
This research received no specific grant from any funding agency.
The authors declare no conflicts of interest regarding the publication of this paper.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Fayemi OS, Giwa AA, Aderanti AE, Adenekan AI (2023) Evaluation of Chemical and Radiological Impacts of Radionuclides in Borehole Waters in Igbajo Town, Osun State, Nigeria. J Phys Chem Biophys. 13:366.
Received: 08-Nov-2023, Manuscript No. JPCB-23-27971; Editor assigned: 10-Nov-2023, Pre QC No. JPCB-23-27971 (PQ); Reviewed: 24-Nov-2023, QC No. JPCB-23-27971; Revised: 01-Dec-2023, Manuscript No. JPCB-23-27971 (R); Published: 08-Dec-2023 , DOI: 10.35248/2161-0398.23.13.366
Copyright: © 2023 Fayemi OS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.