Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Case Report - (2021)Volume 12, Issue 6
Male pattern baldness or androgenetic alopecia in male is a common condition with variable psychosocial impact with some individuals adapting well while others needing therapeutic support. Recently, topical combination serum containing growth factors, copper tripeptide, and Swertia japonica extract was introduced as adjuvant treatment for male pattern baldness. The randomized, single blind, and comparative study was done to assess the effectiveness and tolerability of the combination serum product versus placebo in six-months treatment using nano-fractional radiofrequency in androgenetic alopecia male patients. This pilot study represents the first independent case study of a combination of serum contain copper tripeptide and plant extract Swertia japonica for hair growth. The findings of this case study suggest that this formulation is safe and efficacious as adjuvant therapy for male pattern baldness.
Androgenetic alopecia; Hair loss; Perifollicular pigmentation; Baldness
Male pattern baldness, or androgenetic alopecia, refers to a loss of hair on the scalp, affecting 80% in men and typically occurs later in life [1]. This condition impacts self-image and is a great cause of anxiety and depression in some men. This high prevalence in older men suggests that this form of hair loss may be considered a normal consequence of ageing [2]. The search for treatment results into few drugs of synthetic origin, but side effects associated with them cannot be neglected [3]. Limited treatment options available for male pattern baldness has led to the search for other agents that can provide benefit for this condition. Plant extract formulations are viable alternative to synthetic drugs. Recently, topical combination serum-containing copper tripeptide and Swertia japonica extract was introduced as adjuvant treatment for male pattern baldness. Copper tripeptide or glycyl-L-histidyl-L-lysine, a tripeptide with affinity for copper ions, has been described as a growth factor for a variety of differentiated cells and a modulator of the extracellular matrix. Copper peptide may stimulate hair growth by increasing the proliferation of dermal papilla cells and by preventing their apoptosis [4]. We assessed the effectiveness and tolerability of the combination serum product versus placebo in six months treatment using nano-fractional radiofrequency in androgenetic alopecia male patients. Male pattern androgenetic alopecia is characterized by progressive hair loss from the scalp. It is known that the imbalances of some trace elements play a role in the pathomechanism of many forms of alopecia. Men normally lose their hair when three main factors interact: Genetics, age and hormones.
This study is a randomized, single-blind, comparative study at CELV Dermatology & Aesthetic Clinic in Jakarta, Indonesia. Six male subjects, age between 35 and 65 years, who completed an informed consent procedure with Hamilton-Norwood Scale IV-VI male pattern baldness [5], diagnosed with androgenetic alopecia, confirmed by a dermatologist. Diagnosis androgenetic alopecia was made based on clinical and dermoscopy/ trichoscopy examination (Heine®, Germany). All the subjects were classified into two groups. In the first group, three patients undergo nano-fractional radiofrequency (Venus Viva®, US) following application of fixed combination serum. The other group is three patients undergoing nano-fractional radiofrequency following application with placebo (natrium chloride 0, 9%). All patients followed up every one month for six months session (6 months treatment). The serial conventional photographic examination taken every month during treatment sessions. Clinical outcomes were assessed for pre and post comparison [6]. Additionally, subjects' satisfication for the change in hair growth, and overall improvement was also evaluated using a 5-rating score. The patients 5-score rating score as follows; 1=very dissatisfied, 2=not very satisfied, 3=slightly satisfied, 4=satisfied, 5=very satisfied. Physicians’ satisfaction scores to compare before and after treatment by photographs can be determined by an independent-blinded investigator using a clinical quartile rating scale. The quartile rating scale is as follows: 0=0%-25% improvement, 1=26%-50% improvement, 2=51%-75% improvement, and 3=75% improvement. Adverse effects such as irritation or allergic condition also are determined.
Characteristic trichoscopic features of androgenetic alopecia are hair diameter diversity, perifollicular pigmentation/peripillar sign, and yellow dots as seen from the examination on subjects (Figure 1). Of the total subjects enrolled in the study, 3 active and 3 placebo subjects completed the study [7]. There were no serious adverse events reported, and the mild adverse events included 1 report of scalp itching. These events occurred in active groups, likelihood indicating that the volatile vehicle and not the active was the source of the irritation. The study yielded investigator, subject, and photographic assessments.
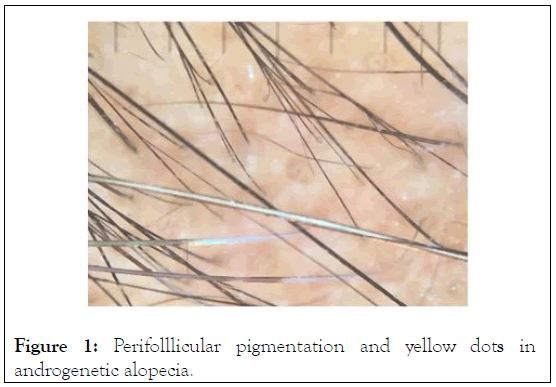
Figure 1: Perifolllicular pigmentation and yellow dots in androgenetic alopecia.
The key assessment was based on the standardized conventional photographs that were evaluated by an independent-blinded investigator for assessment of improvement in hair fullness. On the basis of the independent-blinded investigator assessment of change in the patient’s scalp hair growth from baseline, treatment in active group demonstrated as scale 1 improvement (26%-50%) at the final visit as compared to baseline as shown in (Figures 2 and 3). In contrast, in the placebo group were rated as scale 0 improvement (0%-25%). Nano-fractional radiofrequency used in this study are commonly used for skin rejuvenation and have been shown to induce new collagen and new Extracellular Matrix (ECM) formation in the 3 dermis [8]. When treating scalp skin by radiofrequency device this rejuvenating effect with new ECM may lead to improved anchorage of hairs in the follicles [9,10]. To our knowledge, this study represents the first attempt to evaluate the effects of topically applied serum contain combination copper tripeptide and Swertia japonica extract after nano-fractional radiofrequency in men with Hamilton-Norwood IV-VI male pattern baldness.
Figure 2: Improvement in male pattern baldness as a result of application serum containing copper tripeptide and plant extract Swertia japonica after treatment with nano-fractional radiofrequecy as documented by conventional photography at baseline, 3 months and 6 months of application.
Figure 3: Graphic representation of the results of the patient self-assessment (after treatment completed) of the study group.
This pilot study represents the first independent case study of a combination of serum contain copper tripeptide and plant extract Swertia japonica for hair growth. The findings of this case study suggest that this formulation is safe and efficacious as adjuvant therapy for male pattern baldness. More randomized and multicentric trials with a large number of patients will be required to prove the validity of these results, to obtain these answers, and to get more robust statistical results. Research is also underway to identify additional botanical compounds that may offer useful activity against male pattern baldness.
This study was funded by Highside Corp. in Tokyo, Japan.
Citation: Pamela RD (2021) Evaluation Efficacy of Topically Applied Serum Containing Copper Tripeptide and Swertia japonica Extract in Male Pattern Baldness: A Placebo-Controlled Case Study. J Clin Exp Dermatol Res. 12:590.
Received: 08-Dec-2021 Accepted: 21-Dec-2021 Published: 28-Dec-2021
Copyright: © 2021 Pamela RD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.