
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2024)Volume 14, Issue 1
Objectives: The present study was undertaken to evaluate the acute oral toxicity of hydro-alcoholic leave extract of Annona muricata and to derive the LD50. The purpose of the study was to investigate acute toxicity test of soursop leave extract on Wister rat brain, liver and kidney.
Methods: Acute toxicity studies of hydro alcoholic leave extract of Annona muricata were conducted as per the OECD guidelines 407. The animals were grouped into three test and one control groups. Except for treatment with the test substance, animals in the control group were Liaw led in an identical manner to the test group subjects. The mice were observed until day 7 to determine the LD50 and at the end were terminated to collect the brain, liver and kidney. The organs later were made into histopathology slides. The slides read with light microscope. Data were assessed by one-way ANOVA followed by Tukey’s multiple comparison tests. Values for which starting from p<0.05 was considered as statistically significant.
Results: The acute toxicity study done using OECD 420 guideline revealed that hydro alcoholic leave extract of Annona muricata were safe at 100 mg/kg and produced mortality at 1000 and 2500 mg/kg. On gross examination, body weight, biochemical parameter (Serum Glutamic-Oxaloacetic Transaminase (SGOT), Serum Glutamic Pyruvic Transaminase (SGPT), blood urea) was observed.
Conclusion: The present study concluded the toxicity produced by A. muricata leave in the biochemical parameter and organ (brain, liver and kidney). The LD50 of soursop leave extract is more than 2500 mg/kg BW. This result indicate that the low dose of extract is practically nontoxic and do not damage the brain, liver and kidney.
Acute oral toxicity; Annona muricata; OECD 407; Biochemical parameters; Animal study; Leave extract
Annona muricata L, commonly known as soursop, graviola, guanabana, paw-paw and sirsak, is a member of the Annonaceae family comprising approximately 130 genera and 2300 species [1]. A. muricata is native to the warmest tropical areas in South and North America and is now widely distributed throughout tropical and subtropical parts of the world, including India, Malaysia and Nigeria [2]. A. muricata is an evergreen, terrestrial, erect tree reaching 5-8 m in height and features an open, roundish canopy with large, glossy, dark green leaves. The edible fruits of the tree are large, heart-shaped and green in color, and the diameter varies between 15 and 20 cm [3].
The main chemical compounds (phytochemical) in the soursop leaves are the alkaloid known as acetogenin which contains more than 60 types of alkaloid compounds, including at least 13 alkaloid compounds that have the killing power against cancer cells, even when the cancer cells have developed resistance to modern chemotherapy drugs such as Adriamycin, Vinblastine and Vincristine [4]. Research about the killing power of acetogenin against cancer cells was conducted at Purdue University, West Lafayete, Indiana, USA, in 1997, reported that the acetogenin compounds are potent inhibitors towards enzymatic reactions in the cell membrane by blocking ATP (Adenosine Triphosphate) [5]. Soursops are commonly used in the society. However, there is no certain dosage or measurement for its use. Therefore, it is necessary to conduct toxicity test to measure the safety of the usage of soursop leaves. The acute oral toxicity test is one of the important preclinical trials.
This test is designed to determine the toxic effect of a substance that will occur within a short period of time after oral administration in a certain dose [6]. The quantitative data obtained from acute toxicity test, which is lethal dose 50 (LD50), are able to qualify the toxicity of a substance whether extremely toxic or practically non-toxic. LD50 are also able to predict other toxicology tests, such as sub chronical, and chronical tests. The qualitative data obtained include the clinical manifestations, and mechanisms of toxic effects on various organs such as the liver and kidneys. The liver is the organ where the metabolism and detoxification of various foodstuffs including those containing toxin, while the kidney is a major organ for excretion [7-10].
Preparation of leave extract
The leaves of the plant material are washed and dried in shade. The dried leaves of the plant material are powdered and sieved in a 40 μm mesh. The powdered leaves are extracted with a low polar solvent at 50°C temperatures for 3 hrs resulting in the removal of lipids, fats, oils, and colored pigments. The processed leaf extracts are combined with ethanol and water forming different types of solution. The solutions were extracted by the decoction method with reflux attachment at a temperature below 50°C for 6 hr. The excess solvents are removed by distillation and drying process from the extract at 50°C temperatures for 12 hrs. The dried extracts are mixed in the same ratio to get the desired product. Herein, the leaves of the plant material should have one year and above age. Herein, the polar solvent is selected from but not limited to water, acetic acid, ethanol, and propanol.Animal study
This study population was Wister rats with flowing inclusion criteria: Both sex rats, 180-250 g weight and healthy rats. The animals were maintained on pellet feed with free access to foodand water. They were housed in polypropylene cages, maintained at 21 ± 1°C and 12 h light/dark cycle. The animals were then randomized into four groups with five animals of both the sexes in each group. The experiment will be carried out as per the guidelines of the OECD 407.
Experimental study
The animals were grouped into three test and one control groups. Except for treatment with the test substance, animals in the control group were handled in an identical manner to the test group subjects. Dose levels were selected taking into account any existing toxicity and toxico-kinetic data available for the Annona extract the animals are dosed with test substance daily 7 days each week for a period of 28 days. The maximum volume of liquid that can be administered at one time depends on the size of the test animal. Thus 10 ml/kg was administered to each animal (Table 1).
| Groups | Treatment | Number of animals |
|---|---|---|
| I | Control (normal saline) (10 ml/kg) | 10 |
| II | Therapeutic dose (100 mg/kg ) | 10 |
| III | Dose 2 (1000 mg/kg ) | 10 |
| IV | Dose 3 (2500 mg/kg ) | 10 |
Table 1: Distribution of animals across treatment groups with corresponding dosages.
The highest dose level was chosen with the aim of inducing toxic effects but not death or severe suffering. Thereafter, a descending sequence of dose levels was selected with a view to demonstrating any dosage related response and No-Observed- Adverse Effect Level at the lowest dose level (NOAEL).
The blood collected at termination of the study through retro- orbital plexus of the animals under light ether anesthesia from all groups. The observation period should be 28 days. General clinical observations were made at least once a day, at the same time each day. The health conditions of the animals were recorded on a daily basis. At least twice daily, all animals were observed for morbidity and mortality. Signs noted include, changes in skin, fur, eyes, mucous membranes, occurrence of secretions and excretions and autonomic activity (e.g., lacrimation, piloerection, pupil size, unusual respiratory pattern). Changes in gait, posture and response to handling, stereotypies (e.g., excessive grooming, repetitive circling) or bizarre behavior (e.g., self-mutilation, walking backwards) were also recorded. All animals were weighed once a week.
The animals in first group (therapeutic dose) no rats died during treatment and showed no signs of toxicity. Behavioral changes of rats after treatment were also not present. There were no changes in the nature of stool, urine and eye color. However, weight gains were observed in all animals administered 100 mg/kg/day. It can be stated that at 100 mg, the extract did not interfere with the normal metabolism of animals as corroborated with non- significant difference from animals in vehicle control group. Dose 1000 and 2500 mg/kg/day resulted in decline in body weight of female rats throughout the duration of experiment. ![]()
A total of 20% mortality rate was observed in the third (highest toxic dose) group with symptoms of hypokinesia and Parkinsonism. Clinical observations include lose in grip strength, irregular gait and posture, less than normal activity, difficulty in breathing and slow locomotor activities. Same symptoms were observed in 30% animals from both second (medium toxic dose) and third (highest toxic dose) groups. At higher dose (1000 and 2500 mg/kg/day), the crude extract may have been metabolized to a toxic end product which could interfere with gastric function and decreased food conversion efficiency. The diets were well-accepted by animals treated with 100 mg suggesting AMAE did not possibly cause any alterations in carbohydrate, protein or fat metabolism in these experimental animals. For, animals administered 1000 and 2500 mg extract, the overdose could result in loss of appetite and decrease in body weight in females (Figure 1).
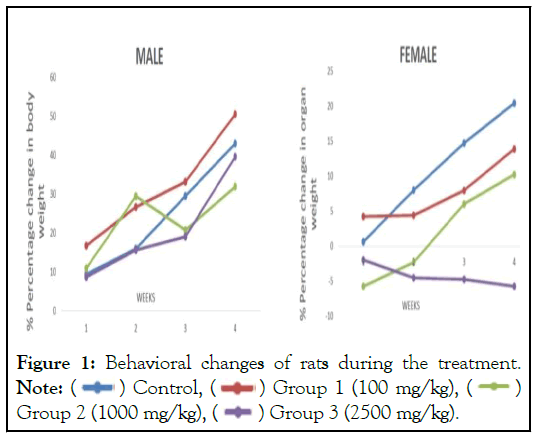
Figure 1: Behavioral changes of rats during the treatment.
Note: 

The liver releases Alanine Aminotransferase (ALT) and an elevation in its plasma concentration is an indicator of liver damage. Any necrosis of liver cells leads to a significant increase in SGOT, SGPT and other enzymes. The results of our study showed significant changes in SGOT, SGPT and ALP levels at high doses suggesting minimal toxicity.
There was a decrease in blood urea and serum creatinine levels in males thus suggesting a kidney protective function. However, in females, there was significant increase in blood urea level only suggesting possible kidney damage, especially by renal infiltration mechanism (Figure 2).
Figure 2: The image showed significant changes in SGOT, SGPT, blood urea level in rat groups.
Histopathology
Full histopathology was carried out on the preserved organs and tissues of all animals in the control and high dose groups. All gross lesions were examined (Figure 3).
Figure 3: Shows full histopathology of the preserved organs and tissues.
The histopathological findings of brain of both the toxic groups (Group II and Group III) showed pyknotic changes, darkening and loss of cells in hippocampal region and cortex suggesting neurodegenerative consequences. Another finding of significance was that the liver of both Group II and Group III (both sexes) showed cytoplasmic vacuolation, increase in sinusoidal gaps and other first degree pathological changes which generally occur due to disturbance in hepatocytic enzymes suggesting minimal liver toxicity caused by the drug at high dose.
Statistical analysis
Data were analyzed using Graph Pad Prism 5 for Windows. The experimental results were expressed as the Mean ± Standard Error Mean (SEM). Data were assessed by one-way ANOVA followed by Tukey’s multiple comparison tests. Values for which starting from p<0.05 was considered as statistically significant.
No mice died during the treatment, change of the nature of mice after getting treatment wasn’t present as well. The criteria of the OECD (Organization for Economic Cooperation and Development) guidelines for the testing of chemicals were met, because of it [11-13]. Soursop leaves excerpt LD50 is lesser than 2000 mg/ kg body weight. This result shows the soursop leaves excerpt is a component that's practically non-toxic to the single oral dosage administration. Further examination on histopathological preparation of the mice’s livers and kidneys also did not indicated necrosis or degeneration. These results are in contrast to studies conducted by Arthur, et al. [14], stating that at high doses, soursop leaves extract causes damage to the kidney tissue. Research conducted by Dayeef, et al. [15], also showed that consumption of soursop leaves extract within 40 days (subchronic) cause kidney damage at the cellular and molecular level. Toxic effects on the kidneys from soursop leaves extract is thought to be the result of a decrease in the amount of caspase 9 and most visible in the cells of the renal tubules.
At high doses, soursop leaves extract caused liver cell necrosis due to the mechanism that inhibits the effect acetogenin mitochondria complex I in hepatocytes. The yield difference raises a new question how security soursop leaves extract when used in high doses in the long term. Therefore, further toxicity studies as subchronic and chronic toxicity tests need to be done to get the complete security data of soursop leaves extract. Various cytotoxic studies conducted with the seeds of A. muricata had revealed that annonaceous acetogenins possessed cytotoxic activity [12,13,16]. Hence, the extraction procedure was selected in a manner to isolate these annonaceous acetogenins. Previous studies have reported the presence of annonaceous acetogenins using the extraction procedure [17]. The authors Chang, et al. [17], have also reported various annonaceous acetogenins in CMAM when analyzed using liquid chromatography-mass spectroscopic analysis in CMAM.
The present toxicity investigation employed a fixed oral dose method as per OECD test guideline 420 for the preliminary AOT evaluation, which provides information on the health hazards that may arise on acute exposure and classify substances according to the globally harmonized system for the classification of chemicals (OECD guideline). In the present study, MAM and CMAM showed mortality at 5000 mg/kg and 2000 mg/kg with congestion of cranial and mesenteric blood vessels and multifocal ecchymosal hemorrhagic lesions. In vitro haemolytic assay also revealed that the fraction was able to induce hemolysis. Annonacin has been reported to deplete the ATP supply in rat striatal neurons and interrupted the transportation of mitochondria to the cell soma which caused the cellular perturbations of tau protein and led to similar characteristics of neurodegenerative diseases [18]. It has also been reported that the fruits of A. muricata with annonacin, as the major annonaceous acetogenin, showed the potential risk for neurodegeneration [19].
The results of the present study also confirm the neurotoxicity. Besides neurotoxicity, the gastrointestinal tract and liver were also affected probably by annonaceous acetogenins. The study revealed no mortality at 300 mg/kg. Although moderate abnormal clinical signs of diarrhea during the initial 3 h following dosing at 300 mg/kg were observed, it did not produce any deleterious effect on body weight gain and gross pathology. Since the acute toxicity study using 420 Guideline revealed that the test substances were safe at 300 mg/ kg, it became imperative to find the estimated oral LD50 of CMAM to derive the pharmacological safe dose as the plant fraction concentrated maximum annonaceous acetogenins. Estimated oral LD50 assessment was done using up-and-down procedure as per OECD test guideline 425. In the study, the LD50 value was estimated to be 1310 mg/kg in rats and 310 mg/kg in mice. The animals dosed at 175 mg/kg survived the 14 day observation period. The research has been carried out with the objective of determining the safety of the association of the hydroalcoholic extract of Curcuma longa rhizome; Cordia fruit, Myrciaria floribunda fruit and Aspidosperma for 28 days. Observations indicate that there were no significant changes in body weight between the groups, nor were there any clinical changes modification of food intake is considered an indicator of toxicity [20-22].
It is concluded that the administration of the hydro alcoholic leaf extract of Annona muricata rats leaves for 28 days, does not present acute and subacute toxicity in low dose, as evidenced by clinical, biochemical and histopathological parameters; Thus, demonstrating the safety of the atomized extract at the tested doses. Hence, from the above findings help us to conclude that Annona hydro alcoholic extract when given at therapeutic or normal dose causes no toxicity on all the major organs in both males and females. While, it is neurodegenerative and it causes minimal liver toxicity when given at high doses.
The authors declare no conflicts of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Chauhan S, Srivastava V (2024) Evaluation Acute Toxicity Studies of Hydro Alcoholic Extract of Soursop Leaves (Annona muricata) on Brain, Liver and Kidney of Wister Rats. J Clin Toxicol. 14:556.
Received: 03-Jan-2024, Manuscript No. JCT-24-28992; Editor assigned: 05-Jan-2024, Pre QC No. JCT-24-28992 (PQ); Reviewed: 19-Jan-2024 Revised: 26-Jan-2024, Manuscript No. JCT-24-28992 (R); Published: 02-Feb-2024 , DOI: 10.35248/2161-0495.24.14.556
Copyright: © 2024 Chauhan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.