Journal of Fertilization: In Vitro - IVF-Worldwide, Reproductive Medicine, Genetics & Stem Cell Biol
Open Access
ISSN: 2375-4508
ISSN: 2375-4508
Research Article - (2024)Volume 12, Issue 2
Objectives: To determine the comparative efficacy of oral Dydrogesterone (DYD) with Vaginal Progesterone (VP)/ Intramuscular Progesterone (IMP) for Luteal-Phase Support (LPS) in women undergoing Frozen Embryo Transfer (FET): By a systematic review and meta-analysis based on all original research published in PubMed, Web of Science, Scopus, CINAHL complete, and Embase up till 26th October 2022. The protocol was registered with Prospero (CRD42022372123 Title: Evaluating the role of oral DYD for luteal-phase support in women undergoing frozen embryo transfer: Systematic review with Meta-analysis. Last Edited: 12/11/2022).
Methods: Following PRISMA 2020 guidelines under a PICOS framework, we included original articles on women undergoing FET, receiving luteal phase support (Population), with either oral DYD (Intervention) or Vaginal Progsesterone (VP)/Intramuscular Progesterone (IMP) (Comparators), measuring pregnancy rate miscarriage rate, live birth rate, patient satisfaction, and side effects (Outcomes)
Results: Of 366 studies identified, 30 full-text articles were selected. Out of these 30 articles, 22 were excluded for varied reasons. Therefore, this FET meta-analysis included 8 articles covering oral DYD as an intervention, (n=3051) and comparators (n=2174) consisting of VP (n=690) and IMP (n=1484). Pooled data from both RCTs and observational studies showed that clinical pregnancy, live births and miscarriage did not differ significantly between oral DYD and VP/IMP groups. Women on oral DYD reported significantly higher patient satisfaction scores than on VP (4.09 ± 0.96 vs. 3.36 ± 1.23; P=0.001). Likewise, women experiencing at least one side effect were the fewest among those who received DYD (7.7% on DYD, 16.4% on VP, and 52.3% on IMP). Only 1.9% on DYD experienced moderate to severe side effects vs. 5.5% on VP and 29.5% on IMP. Side effects forced eight women to discontinue IM progesterone.
Conclusions: Fewer side effects and demonstration of non-inferiority in clinical outcomes make oral DYD a better option than VP and IM progesterone for LPS in women undergoing FET procedure for IVF.
Assisted reproduction techniques; Frozen thawed embryo transfer; Luteal phase support; Progesterone; Dydrogesterone
Cryopreservation substantially facilitates Frozen-thawed Embryo Transfer (FET) in Artificial Reproductive Technologies (ART) [1]. Better cryopreservation strategies, progress in vitrification techniques, better safety profiles and favorable pregnancy outcomes have together increased the practice of FET over the years. Elective transfer of frozen embryos reduces the risk of Ovarian Hyperstimulation Syndrome (OHSS) and multiple pregnancies. Such a strategy also improves the success rate of Embryo Transfer (ET) and cumulative pregnancy outcomes [2,3]. FET accounts for about a fourth of ART births today [1].
Clinical Pregnancy Rates (CPR) was similar for In Vitro Fertilization (IVF) carried out either by fresh or frozen-thawed embryo transfer. Synchronizing the endometrium for the preimplantation cycle is the essential precondition for a successful FET cycle. Progesterone prepares the endometrium to receive the embryo and reduces abortion in the initial phase of pregnancy. The quality of the embryo is critical for maintaining a good Live Birth Rate (LBR) in FET cycles. Considering the wide variation in the prevalence (3.7%-20%) of (LPD) in infertile subjects, [4], providing optimal Luteal Phase Support (LPS) is critical for enabling the success of FET cycles [5]. Appropriate LPS significantly improves clinical pregnancy outcomes and increases the chances of live births in IVF treatment cycles. VP delivers a high concentration for direct action on the endometrium during LPS. However, VP has multiple limitations which include the need for multiple daily applications, vaginal irritation, and vaginal discharge. Moreover, vaginal progesterone cannot be used during vaginal bleeding [6,7]. LPS has become the standard practice in ART because suboptimal LPS impair the success rate in FET cycles [8].
Dydrogesterone (DYD) is structurally and pharmacologically similar to progesterone with the added advantage of oral bioavailability [5]. DYD is an optical isomer of progesterone that enjoys greater stability in formulations and improved oral bioavailability. DYD does not adversely impact the mother, fetal development, or birth outcomes. Two randomized controlled trials namely Lotus I and Lotus II, published in 2017 and 2018, [9,10] have already established the non-inferiority of oral DYD over vaginal progesterone in fresh IVF cycles. Following this, patients’ demand for oral DYD (for LPS) over other alternatives has increased. However, there are neither narratives nor systematic reviews, with meta-analyses, summing up the multiple results obtained from comparing oral DYD with VP and IMP in FET cycles. This is the first systematic review with meta-analysis comparing the prevailing status of oral DYD with IMP and VP in FET cycles.
The systematic review protocol was registered in the international Prospective Register of Systematic Reviews (PROSPERO) under the identification number-CRD42022372123 (date: 12th November 2022). We conducted the review as per the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2020 guidelines under a PICOS framework-We included original articles (study design) on women undergoing FET, receiving luteal phase support (Population), with either oral DYD (Intervention) or VP/IMP (Comparator), and measuring primary outcomes such as pregnancy rate and miscarriage rate. Live birth rate, patient satisfaction, and side effects were the secondary outcomes.
We reviewed Randomized Controlled Trials (RCTs) and observational studies comparing oral DYD vs. VP/IMP for LPS in women undergoing FET. There was no language restriction. Eligible articles in languages other than English were translated by a Mandarin translator. Pre-clinical studies, editorials, case reports, review articles, conference proceedings, abstract-only papers, and studies where the outcome could not be assessed were excluded.
Types of interventions
Interventional group constituted women who received oral DYD against VP/IMP as controls for luteal-phase support in women undergoing FET.
We evaluated both the efficacy and safety outcomes of oral DYD compared to VP/IMP for LPS in women undergoing FET. Ongoing pregnancy, live birth rate, and miscarriage rates were the efficacy outcomes. Patient satisfaction and side effects were the safety outcomes.
Search strategy
An extensive literature search was performed to select relevant articles published in PubMed, Web of Science, CINHAL, Scopus, and Embase on 26th October 2022. The search strategy was designed using the keywords and Medical Subject Headings (MeSH) terms like ‘In Vitro Fertilization’, ‘test tube fertilization’, ‘IVF’, ‘ICSI’, ‘embryo’, ‘blastocyst’, ‘oocyte’, ‘egg’, ‘embryo transfer, ‘HRT-FET’, ‘Luteal’, ‘luteal phase support’, ‘DYD’, ‘Duphaston’, ‘isopregnenone’, ‘dydrogesterone’, ‘progesterone’ using ‘AND’ and ‘OR’.
Eligibility and study selection
The studies were screened by title and abstracts, followed by full-text articles based on predefined criteria. Two independent reviewers (Dr. Tejaswini Baral and Dr. Shilia Jacob) performed the study selection and data extraction. Disagreements were resolved by mutual consultation with a third reviewer (Dr. MK Unnikrishnan). After the initial search, all references were downloaded to Microsoft Excel.
As we had no restriction on the languages, 2 out of 8 selected articles that were originally in Mandarin were translated into English by a Mandarin translator [11,12]. Data from the included studies were extracted into a pre-framed data extraction sheet. The following variables were extracted: Author names, year of publication, place of study, study design, patient demographic characteristics, number of patients in cases/control, the reason for infertility, FET outcomes in terms of pregnancy rate, miscarriage rate, live birth rates, patient satisfaction, and side-effects.
Data extraction and quality assessment
The Cochrane Risk-Of-Bias (CROB) tool was used to assess the quality of RCTs and the Newcastle-Ottawa Scale (NOS) for observational studies (case-control and cohort studies). Two independent reviewers (Dr. Baral and Dr. Jacob) evaluated the methodological quality of included studies, and any disagreements between the reviewers were settled through consensus/discussion with a third reviewer (Dr. MK Unnikrishnan). Cochrane risk-ofbias tools were used to assess the quality of RCTs. The Newcastle- Ottawa Scale (NOS) was used for observational studies (such as case-control and cohort studies), and Joanna Briggs Institute (JBI) critical appraisal checklist for cross-sectional studies. Two independent reviewers (Dr. Baral and Dr. Jacob) performed the quality assessment, and any disagreements between the reviewers were settled through consensus or by a discussion with a third reviewer (Dr. MK Unnikrishnan).
Statistical analysis
Review Manager Software (RMS) (RevMan, version 5.3 for Windows; The Cochrane Collaboration, Oxford, UK) [13], was used to compare outcomes in women on oral DYD and VP/IMP. Risk Ratio (RR) and 95% Confidence Interval (95% CI) values were calculated. The I2 statistic was used to assess the statistical heterogeneity of data. If I2 ≤ 50% or P ≥ 0.10, the fixed-effects model was used, whereas if I2>50% or P ≤ 0.10, the randomeffects model was used. Publication bias was detected using funnel plots, generated using RevMan ver. 5.3.
Study characteristics
A total of 366 RCTs and observational studies from the years 1970 to 2022 were first identified for evaluation. Pre-clinical studies, editorials, case reports, review articles, conference proceedings, abstract-only papers, and studies where the outcome could not be assessed were excluded. Only original articles on women undergoing FET, receiving luteal phase support, and receiving either oral dydrogesterone or comparator vaginal or IM progesterone were included.
Based on the criteria described in the methods, 30 publications were eligible for full-text evaluation. Out of these 30 articles, for 22 articles, please find Table 1 below listing the number of screened articles and their reason for exclusion from the FET Meta-analysis. Finally, eight full-text articles were included in this meta-analysis, of which five were RCTs and the remaining were retrospective observational studies. A total of 3051 women received oral DYD, 690 received VP, and 1484 received IMP. See PRISMA flow chart in Figure 1.
| S.No | Articles with title and abs | Reason |
|---|---|---|
| 1 | 14 | Does not include FET. |
| 2 | 5 | Oral DYD given as a combination. |
| 3 | 2 | Does not include oral DYD as an intervention. |
| 4 | 1 | Could not be retrieved by our literature vendor. |
Table 1: Reasons for exclusion of 22 out of 30 selected articles.
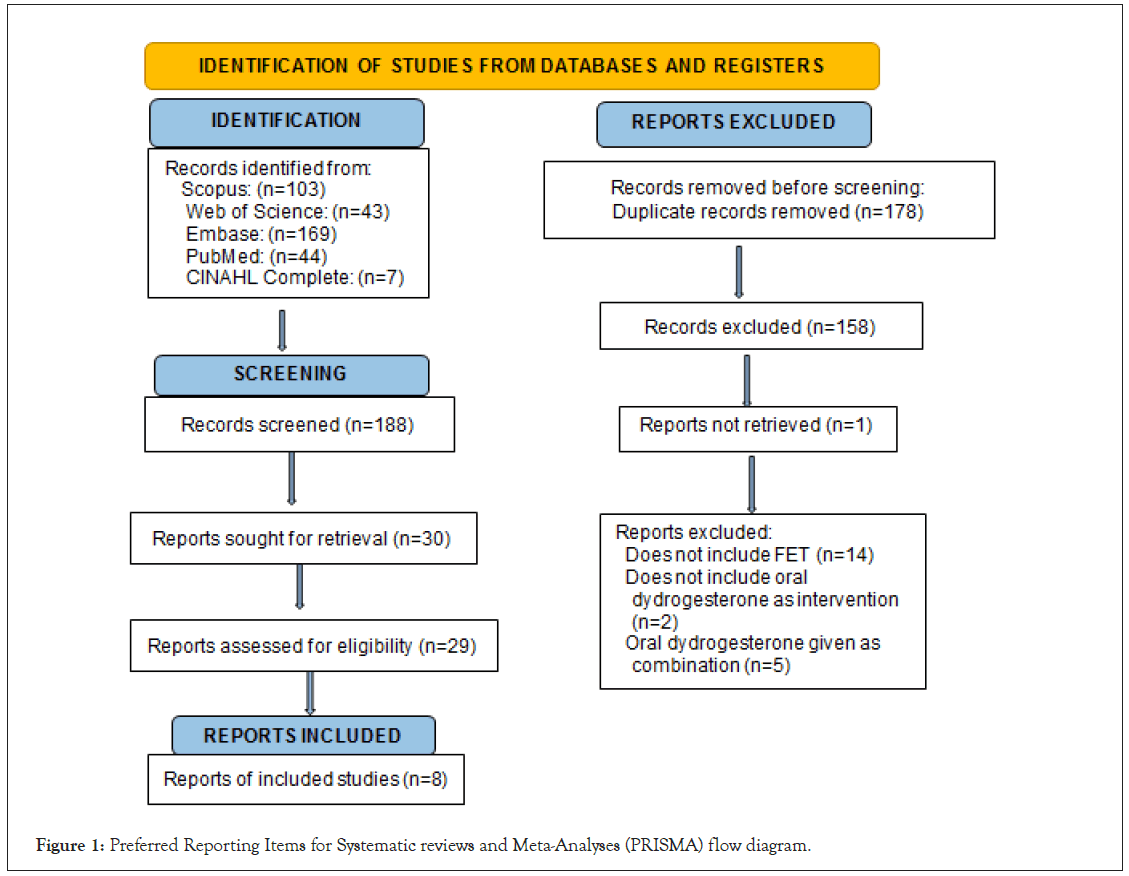
Figure 1: Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram.
The study characteristics have been summarized in Table 2. There were 3051 women in the intervention/exposure group (oral DYD) and 2174 women in the control group (VP and IMP), with a mean age between 31.70 ± 6.48 to 35.5 ± 6.4.
| References | Country | Study design | Period of enrolment | Study size (N) | Intervention/ exposure (number of participants) |
Control (number of participants) | Age (in years) | BMI |
|---|---|---|---|---|---|---|---|---|
| Rashidi BH, et al. | Iran | RCT | January 2015 to May 2016 |
180 | Oral DYD: 20 mg BD (N=60) | VP: 400 mg BD (N=60); IM P: 50 mg BD (N=60) | Oral DYD: 31.70 ± 6.48, VP: 33.27 ± 5.69, IM P: 32.05 ± 6.25 | Oral DYD: 25.16 ± 2.89, VP: 24.56 ± 3.05, IM P: 25.19 ± 3.57 |
| Zarei A, et al. | Iran | RCT | December 2014 to March 2015 | 200 | Oral DYD: 10 mg BD (N=100) | VP: 400 mg BD (N=100) | Oral DYD: 32.90 ± 5.10, VP: 33.51 ± 5.20 | NA |
| Ozer G, et al. | Turkey | RCT | January 2019 to August 2019 | 134 | Oral DYD: 10 mg TID (N=67) | VP: 8% gel OD (N=67) | Oral DYD: 31.88 ± 5.20, VP: 32.40 ± 3.74 | Oral DYD: 24.44 ± 4.85, VP: 23.23 ± 3.88 |
| Pabuccu E, et al. | Turkey | Pilot RCT | June 2021 to April 2022 | 151 | Oral DYD: 20 mg BD (N=52) | VP: 8% gel BD (N=55); IM P: 100 mg OD (N=44) |
Oral DYD: 32.8 ± 4.2, VP: 32.3 ± 4.4, IM P: 33.8 ± 4.8 | Oral DYD: 22.0 ± 2.3, VP: 22.8 ± 2.2, IM P: 22.3 ± 2.2 |
| Macedo LC, et al. | Brazil | RCT | September 2019 to February 2021 | 73 | Oral DYD: 40 mg/day (N=36) | VP: 800 mg/day (N=37) | Oral DYD: 34.1 ± 4.4, VP: 32.3 ± 4.3 | Oral DYD: 25.2 ± 5.0, VP: 26.5 ± 5.7 |
| Atzmon Y, et al. | Israel | Retrospective | January 2018 to December 2019 | 599 | Oral DYD: 10 mg TID (N=226) | VP: Endometrin 100 mg TID, or 8% gel TID (N=373) | Oral DYD: 34.3 ± 6.1, VP: 35.5 ± 6.4 | Oral DYD: 25.5 ± 5.2, VP: 25.8 ± 5.9 |
| Yang R, et al. | China | Retrospective | January 2011 to March 2013 | 2248 | Oral DYD: 20 mg BD (N=1967) | IM P: 40 mg OD (N=281) | Oral DYD: 32 ± 4, IM P: 33 ± 4 |
NA |
| Guo W, et al. | China | Retrospective case-control | January 2010 to September 2011 | 1643 | Oral DYD: 10 mg TID, or 10 mg q.i.d. (N=543) | IM P: 60 mg OD (N=1100) | NA | NA |
Table 2: Study characteristics.
Risk of bias within studies
The risk of bias of the five RCTs was performed using CROB (Figure 2). All the studies except Macedo, et al. [14], reported the method of randomization. The method of allocation concealment and blinding of participants, personnel, and outcome assessment was reported only by Rashidi, et al. [15]. The attrition bias was high for Ozer, et al. [16], and Pabuccu, et al. [17], because they could not meet the desired sample size. The Newcastle Ottawa Scale (NOS) for observational studies showed that Guo, et al. [11], Yang, et al. [12], and Atzmon, et al. [18] were good-quality studies (final scores 6 to 8).
Figure 2: The risk of bias analysis of the five RCTs was performed using Cochrane Risk of Bias (CROB) tool. Note:  High risk of bias is
denoted by red,
High risk of bias is
denoted by red,  Low risk of bias is denoted by green,
Low risk of bias is denoted by green,  Unclear risk of bias is denoted by yellow.
Unclear risk of bias is denoted by yellow.
Clinical pregnancy: A total of six studies, with 1231 women, compared oral DYD with VP for LPS in women undergoing FET. The combined data from both RCTs and observational studies showed clinical pregnancy was similar in both groups as shown in Figure 3 [RR=1.06, 95% CI: 0.92-1.22; I2=23%, P=0.40]. Similar results were observed with pooled analysis of RCTs [RR=0.98, 95% CI: 0.82, 1.17; I2=34%, P=0.86].
Figure 3: Clinical pregnancy data from both RCTs and observational studies comparing VP to oral DYD.
A total of four studies with (n=4106) compared oral DYD with IMP for LPS in women undergoing FET. The combined data from both RCTs and observational studies showed clinical pregnancy was similar in both groups [RR=1.07, 95% CI: 0.92- 1.17; I2=0%, P=0.20]. Similar results were observed with pooled analysis of RCTs [RR=0.97, 95% CI: 0.71, 1.33; I2=0%, P=0.87] and observational studies [RR=1.07, 95% CI: 0.97, 1.19; I2=0%, P=0.17].
Live births: Two RCTs, with 225 women, compared oral DYD with VP for LPS in women undergoing FET. The live births were similar in both groups as shown [RR=1.04, 95% CI: 0.72-1.51; I2=0%, P=0.82].
Three studies, with 2463 women, compared oral DYD with IMP for LPS in women undergoing FET. The combined data from both RCTs and observational studies showed live birth was similar in both groups in Figure 4 [RR=1.10, 95% CI: 0.93- 1.30; I2=0%, P=0.29]. Similar results were observed with pooled analysis of RCTs [RR=0.93, 95% CI: 0.65, 1.34; I2=0%, P=0.70].
Figure 4: Live birth data from both RCTs and observational studies comparing IMP to oral DYD.
Miscarriages: Six studies, with 443 women, compared oral DYD with VP for LPS in women undergoing FET. The combined data from both RCTs and observational studies showed miscarriages were similar in both groups in Figure 5 [RR=0.85, 95% CI: 0.62- 1.15; I2=34%, P=0.28]. Similar results were observed with pooled analysis of RCTs [RR=1.59, 95% CI: 0.83, 3.03; I2=0%, P=0.16].
Figure 5: Miscarriage data from both RCTs and observational studies comparing VP to oral DYD.
Four studies with 1642 women compared oral DYD with IMP for LPS in women undergoing FET. The pooled analysis showed miscarriages were similar in both groups in Figure 6 [RR=0.93, 95% CI: 0.71-1.23; I2=0%, P=0.62]. Similar results were observed with pooled analysis of RCTs [RR=1.87, 95% CI: 0.48, 7.24; I2=0%, P=0.36] and observational studies [RR=0.90, 95% CI: 0.68, 1.19; I2=0%, P=0.45].
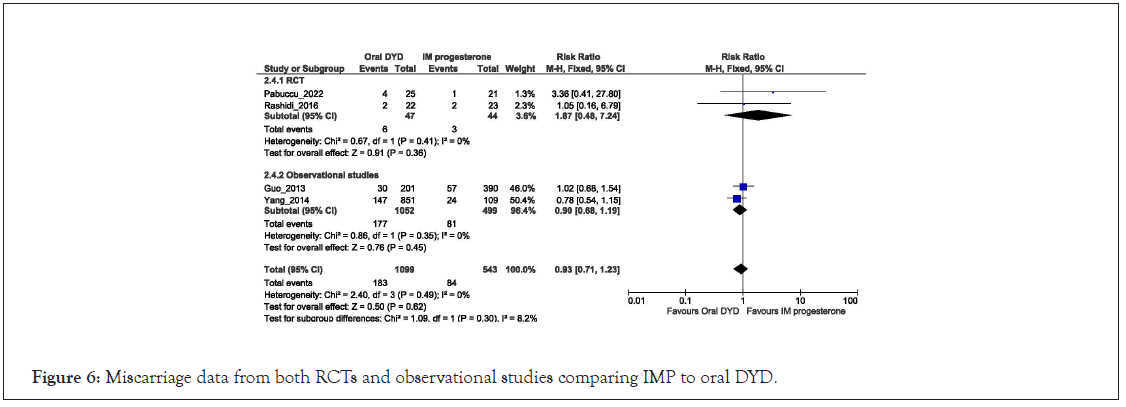
Figure 6: Miscarriage data from both RCTs and observational studies comparing IMP to oral DYD.
Safety and patient satisfaction: Reports by compared treatmentrelated adverse effects and patient satisfaction between women receiving oral DYD and VP. The common adverse effects associated with VP were vaginal discharge (76.9%), preventing sexual intercourse (50%), and vaginal irritation (44.6%), and those on oral DYD were somnolence (38.5%), mastalgia (36.9%), and flatulence (32.8%). A significantly higher patient satisfaction score was observed in women on oral DYD than on VP (4.09 ± 0.96 vs. 3.36 ± 1.23; P=0.001) [16]. Pabuccu, et al. reported that side effects forced 8 women to discontinue IMP and shift to an alternate protocol [17].
Publication bias
We did not evaluate publication bias using the funnel plot because fewer than ten studies were included. Therefore, the statistical estimation using Egger’s or Begg's test was not performed.
Cumulative evidence over many years, generated from many peerreviewed publications, successfully endorses the non-inferiority of oral DYD over VP and IMP for LPS. While the number of live births, clinical pregnancies, and miscarriages do not significantly differ between the three modes of LPS, viz DYD, VP and IMP, a closer look at individual studies reveals more interesting details. For instance, in one of the most recent articles, Atzmon, et al. [18], 2021 expanded the non-inferiority of DYD (over VP/IMP) in not only natural but artificial FET cycles too [18]. Atzmon, et al. [18], 2021 also adds a note implying that ‘DYD might replace vaginal progesterone as the standard of care in FET, including artificial FET’ [18]. Likewise, there are multiple studies echoing similar outcomes.
DYD was synthesized way back in the 1950’s and first introduced to the market in 1961, even before IVF was ever contemplated. In other words, DYD was not introduced with LPS in mind. DYD is structurally related to natural progesterone but enjoys the advantage of much better oral bioavailability. In other words, DYD was identified and promoted to its current status, not by marketing by pharmaceutical industries, but by cumulative clinical experience and systematic research.
To begin with, more successful outcomes were reported with IM progestogens. Vaginal progesterone has demonstrated better implantation, delivery, and live birth than sustained-release progesterone injections. Europe prefers vaginal suppositories because consequent to high systemic absorption by the IM route, the vaginal route was presumed less likely to suppress the hypothalamus-pituitary-ovarian axis. Consequently, the vaginal route was expected to interfere less with endogenous corpus luteum function. However, in the absence of a functional corpus luteum in frozen embryo cycles (unlike fresh embryo transfer cycles) lower systemic progesterone concentrations may not offer any advantage. Thus, a combination of good oral bioavailability, better patient compliance and validated efficacy gradually tilted in favor of oral DYD [19-21].
Interestingly, the only study that does not favor DYD is the RCT by Zairei, et al. 2017 [22] from Iran, which employed a suboptimal dose of DYD, viz 10 mg twice daily. Nevertheless, the same study employs 400 mg of vaginal progesterone as a comparator, the dose employed by most other studies. In other words, Zairie, et al. [22], compares a suboptimal dose of DYD with the optimum dose of vaginal progesterone, which clearly explains the anomalous finding. None of the other studies included in this review attempts to employ DYD at such low doses. Interestingly, if the Zairei, et al. [22], study is excluded from the forest plots of the metanalysis, live birth rates, clinical pregnancy rates and miscarriage rates tend to favor DYD substantially. For instance, miscarriages in women taking oral DYD fell from 22.2% (45 events per 203) to 16.6% (183 events per 1099) when the data from Zairie, et al. [22], was excluded. Likewise, clinical pregnancy improved from 37.5% (203 events per 541) to 44% (194 events per 441) when data from Zairie, et al. [22], was excluded. The above results also strongly validate the dosing regimen introduced previously and supported by Lotus trials.
Patki, et al. and Pawar, et al. 2007 [23], were among the first to confirm that increasing the oral dose of DYD from 20 mg to 30 mg daily improved pregnancy outcomes in a statistically significant manner. The dose employed by Zairie, et al. 2017 did not employ the dose suggested by Patki, et al. and Pawar, et al. despite concrete evidence from previous reports employing 30 mg DYF for LPS with pregnancy rates similar to Micronized progesterone. Standardizing the effective dose of DYD at 30 mg paved the way to Lotus trials 1 and 2, [9,10], the largest trials ever conducted in infertility and ART. Likewise, another study by Mirza, et al. [24], also describes a meta-analysis supporting the use of DYD in early pregnancy.
More interestingly, Zairie, et al. [22], also explicitly suggests a combination of oral DYD and GnRH-alpha or hCG, possibly because of the lower doses of DYD employed, particularly in women who suffered vaginal irritation and discharge upon receiving VP. While acknowledging the adverse effects of progesterone by the vaginal route, authors do consider DYD as an alternative but are possibly prejudiced by the modest outcomes of employing suboptimal doses (10 mg BD) of DYD.
Quite in contrast, one of the largest studies included in this review, (n=2248) comparing IM progesterone and oral DYD, with as many as 1967 receiving oral DYD, has provided very encouraging outcomes in favor of oral DYD. Not only were the clinical pregnancy rates significantly higher (43.78% vs. 34.38%, p<0.05) in the oral DYD group, miscarriage rates (16.54% vs. 29.55% p<0.05) were significantly lower too. Guo, et al. [11], has shown that oral DYD also increased live births by a significant proportion (34.16% vs. 23.44%) [11].
The data extracted in the current review serves as a direct testimony to the popularity DYD already enjoys. Of the subjects covered by articles included in the current meta-analysis, 3051 received oral DYD, 690 received vaginal progesterone, and 1484 received IM progesterone. Oral DYD is the single most popular option for LPS for women undergoing frozen embryo transfer since it caused the lowest number of side effects. Pabuccu, et al. [17], reported that 8 women discontinued IMP due to side effects. More importantly, oral DYD caused the least side effects (7.7%) as opposed to, 16.4% on VP, and 52.3% on IMP. Only 1.9% on oral DYD suffered moderate to severe side effects as against 5.5% on VP and 29.5% on IMP [17]. Patient satisfaction scores were in favor of oral DYD as against VP (4.09 ± 0.96 in DYD vs. 3.36 ± 1.23 in VP) with a statistical significance of p<0.001. Unequivocal findings on fewer side effects could be the major reason for the rising popularity of oral DYD. In the context of LPS with progestogens, Rashidi, et al. 2016 [15], mention that the USA prescribes IM injections [25]. Although DYD was introduced in the USA as Gynorest, it was later discontinued [26].
Interestingly, DYD was not discontinued for reasons of safety or efficacy. It is important to note that, FDA explicitly states that “federal register determination that the product was not discontinued or withdrawn for safety or effectiveness reasons”. The USA is indeed the global leader in guiding the global pharmaceutical policy, chiefly on account of the size of its pharmaceutical market. In the absence of any business incentive to popularise DYD, (a drug so old, off-patent, and outside the US market) financial conflicts of interest are unlikely to exaggerate the clinical value of DYD in peer-reviewed publications.
The authors are grateful to Dr. MK Unnikrishnan, Tejaswini Baral and Shilia Jacob for conducting the meta-analysis.
Ameet Patki and Hrishikesh Pai are co-first authors.
This study has received funding from Mankind Pharma Ltd.
[Crossref][Google Scholar][PubMed]
Citation: Patki A, Pai H, David S, Duru S, Malik S, Rao K (2023) Evaluating the Role of Oral Dydrogesterone for Luteal-Phase Support in Women Undergoing Frozen Embryo Transfer: Systematic Review with Meta-Analysis. J Fertil In vitro IVF World w Reprod Med Genet Stem Cell Biol.11:295.
Received: 07-Mar-2023, Manuscript No. JFIV-23-22070; Editor assigned: 09-Mar-2023, Pre QC No. JFIV-23-22070 (PQ); Reviewed: 23-Mar-2023, QC No. JFIV-23-22070; Revised: 30-Mar-2023, Manuscript No. JFIV-23-22070 (R); Published: 06-Apr-2023 , DOI: 10.35248/2375-4508.24.12.295
Copyright: © 2023 Patki A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited