Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Research Article - (2020)Volume 9, Issue 6
Background: Menopause is associated with increases in sleep-related complaints, including insomnia and mood disorders with associated sleep disruption. Decreased sleep efficiency seen on the polysomnography (PSG) may be related to menopause, OSA, or aging. To identify and treat a new pattern of problems that present with the onset of menopause, sleep physicians should have a better understanding of the effect of OSA treatment with CPAP on quality of life among menopausal women. Therefore, in this study, we hypothesized that sleep architecture improvement with the treatment of OSA would result in subjective improvements in sleep quality in postmenopausal women as assessed by the Post PSG Sleep Assessment (PPSA).
Method: In this study, we prospectively analyzed 49 menopausal women diagnosed with OSA presenting to the George Washington University’s Medical Faculty Associates, Center for Sleep Disorders. From 2012 to 2016, this sample of responders was invited to undergo in-laboratory polysomnography. Patients were treated with continuous positive airway pressure (CPAP). Pre-treatment SF-36, Hamilton rating scales (HAM-D) for depression, insomnia severity index, Epworth sleepiness scale (ESS), and MRS scores were compared with three-month post-treatment scores with Wilcoxon signed-rank test.
Result: During the recruitment period, 60 women underwent polysomnography and were diagnosed with moderate to severe sleep apnea. During the initial follow up visit, 49 women met the eligibility criteria for the study. There was a trend for higher average Epworth Sleepiness Scale (ESS) in patients who were non-compliant to the CPAP treatment (Using the Medicare adherence criteria of ≥ 4 h of use on 70% of nights) than the individuals who adhere to the CPAP treatment. (7.29 versus 6.0 respectively, p< .849).
Conclusion: Although findings show that compliance to Obstructive Sleep Apnea (as assessed by AHI) treatment with CPAP, was unrelated to the severity of menopausal symptoms, there is good evidence that treating OSA improves depression with OSA-related symptoms (i.e., daytime sleepiness, cognitive deficits, etc.). Overall, this study shows that sleep apnea symptoms are more severely expressed by OSA patients who are non-compliant with CPAP treatment.
Obstructive sleep apnea; Postmenopausal symptoms; Depression; Quality of life; Continuous positive airway pressure; Epworth sleepiness scale; Hamilton rating scale
Menopause is characterized by several changes in endogenous hormones due to a depletion of ovarian follicles [1]. It is accompanied by a significant decline in sex hormone levels, giving rise to distressing menopausal symptoms, including hot flashes, night sweats, mood disorders, sleep disturbances, urogenital disorders, bone loss, and metabolic problems. These can have a significant impact on the quality of life. The Study of Women's Health across the Nation (SWAN) [2] found that progression through the menopausal transition was associated with increasing self-reported sleep disturbances. Sleep disturbances during the menopause have been ascribed to several factors: physiological changes of aging, menopausal-related symptoms, stress, mood symptoms (e.g., depression and anxiety), and chronic health issues. Menopause is also associated with an independent risk for depression/depressive symptoms [3] that may impact sleep and quality of life.
Prevalence of another sleep disorder, obstructive sleep apnea (OSA), is increased after menopause [4,5]. OSA is characterized by recurrent collapses of the upper airway during sleep, and is associated with intermittent hypoxia, sleep fragmentation, surges of sympathetic tone, and oxidative stress, finally resulting in increased cardiovascular risk and excessive daytime sleepiness [5-7]. In the general population, OSA treatment with continuous positive airway pressure (CPAP) is associated with improvement in daytime sleepiness and quality of life [8]. However, the impact of OSA treatment on post-menopausal symptoms, including depression and daytime sleepiness, is not known.
The Menopause Rating Scale (MRS) is a standardized, reliable, and validated self-administered scale used to evaluate the severity of symptoms over time, and assess quality of life in post-menopausal women [9]. We hypothesized that treatment of OSA with CPAP in postmenopausal women would lead to improvements in MRS scores, in addition to having an impact on depression and excessive daytime sleepiness in this population.
We conducted a prospective, cohort study in post-menopausal women with moderate to severe newly diagnosed OSA presenting to the George Washington University (GW) Medical Faculty Associates Center for Sleep Disorders. Participants were approached for participation at the time of diagnosis by polysomnography. Data were collected by the authors via questionnaire at initial and subsequent visits. Patients were consented only if they had opted for CPAP for the treatment of sleep apnea. Baseline Epworth sleepiness scale (ESS), Menopausal Rating Scale (MRS) scores and Hamilton rating scale (HAM-D) for depression were obtained prior to starting CPAP therapy and again after 3 months on a followup visit, when a compliance download of their CPAP use was also obtained. Written informed consent was obtained from all patients before enrollment. The study was approved by The George Washington University Institutional Review Board (IRB#031209).
All women above the age of 42 years who were menopausal were eligible to participate. Only women with no self-reported menses for greater than one year were defined as menopausal and considered eligible for participating in the study. Women who have regular menstruation (defined as cycles that vary in length by no more than five days and vary in interval between 23 and 35 days) were defined as premenopausal and excluded from the study. Women with irregular menstrual cycles (defined as cycles lasting <23 days or >35 days or varying by more than five days) or with no menstrual bleeding for less than one year were defined as peri-menopausal and also excluded. We did not exclude women if they were actively taking hormone replacement therapy or antidepressants. However, we excluded patients who were diagnosed with major depression that had initiated a new antidepressant medication or switched to a different dose of their usual antidepressant within six months, or during this study period.
All participants had been diagnosed with moderate to severe sleep apnea based on the results of an in-lab polysomnography (PSG) conducted at The Center for Sleep Disorders at the Medical Faculty Associates at The George Washington University. The PSG was conducted using a Respironics Alice 6 LDXN Sleep Diagnostic System (Philips Respironics, Murrysville, PA). A standard set of clinical measurements were acquired during the PSG, including the ECG, EEG, oxyhemoglobin saturation, and respiratory airflow. Registered polysomnographic technologists (RPSGT) manually scored the PSG data, including identification of sleep stages, apneic and hypopneic events, and arousals using standard criteria [10]. Hypopnea was defined as a reduction in ventilation of greater than 30% that lasted for at least 10 seconds and resulted in a decrease in arterial saturation of 4% or more [10]. Apnea was defined as a reduction in the peak signal excursion of greater than 90% and lasting at least 10 seconds. The severity of OSA was defined by the apnea-hypopnea index (AHI) (total number of apneas and hypopneas during the study divided by the hours of sleep). An AHI of <5/hr is normal; AHI between 5-15/hr is mild disease; AHI between 15-30/hr is moderate and AHI >30/hr is severe disease [11].
Baseline demographic data was obtained from all participants, including age, race and body mass index (BMI). In addition, the participants completed the ESS, MRS, and HAM-D at baseline, and again on follow-up visit.
The Epworth Sleepiness Scale (ESS) is a self-administered questionnaire with 8 questions. Respondents are asked to rate, on a 4-point scale (0-3), their usual chances of dozing off or falling asleep while engaged in eight different activities. Most people engage in those activities at least occasionally, although not necessarily every day. The ESS score (the sum of 8 item scores, 0-3) can range from 0 to 24. The higher the ESS score, the higher that person’s average sleep propensity in daily life (ASP), or their ‘daytime sleepiness’. The questionnaire takes no more than 2 or 3 minutes to answer.
Psychometric properties of the ESS have been extensively investigated. The internal consistency of responses to the eight questions has been tested by Cronbach’s alpha (mean=0.82) in multiple investigations [12,13].
The Menopause Rating Scale (MRS) is a questionnaire evaluating the severity of 11 symptom categories related to menopause on a scale from 0 (no symptoms) to 4 (extremely severe). These include such symptoms as hot flashes, heart discomfort, trouble sleeping, depressive feelings, irritability, sexual problems, vaginal dryness, mental and physical exhaustion, joint and muscular pain, and more. Women assign a number next to each of the eleven symptoms identified on the questionnaire. The total score of the MRS ranges between 0 (asymptomatic) and 44 (highest degree of complaints) [14]. The reliability and validity of the MRS are well-studied in extensive multinational surveys in 2001-2002. Reliability measures were found to be acceptable across nine countries. Cronbach’s alpha ranged between 0.8 and 0.96 across Europe, North and Latin America, and Asia [15].
The Hamilton Depression Rating Scale (HAM-D) is the most widely used clinician-administered depression assessment scale. It is a multiple item questionnaire used to provide an indication of depression, and as a guide to evaluate recovery. A score of 0-7 is considered normal, 8-13 (mild) 14-18 (moderate), 19-22 (severe), and >23 (very severe) depression. The validity of the HAM-D has been repeatedly investigated in general populations. Bagby et al. performed a comprehensive review and collogues examined the psychometric properties of the HAM-D in 70 published studies [16]. The results of their investigation found acceptable internal consistency and discriminant validity. Cronbach’s alpha (mean=0.70) [16].
We defined CPAP compliance per the Center for Medicare and Medicaid Services (CMMS) guidelines, where use of CPAP device for at least 4 hours a night on at least 70% of the nights is considered compliant. Compliance download was obtained from the subject’s CPAP unit at the time of the follow-up visit. Analyses were conducted using R v4.0.2 (RCore Team, Austria 2020) [17]. Descriptive statistics were calculated as medians and IQR of continuous variables due to an assumption of nonnormality, and frequencies reported for categorical variables.
Baseline characteristics are listed in Table 1. During the recruitment period, post-menopausal women underwent polysomnography and were diagnosed with moderate to severe sleep apnea. During the initial visit 49 women met eligibility criteria for the study. Among the participants, 19 women were excluded because either did not start CPAP treatment or they did not have time to participate. A total of 30 women provided informed consent and completed baseline questionnaires for the study. A further eight women were excluded either because they were noncompliant with their treatment or because we were unable to verify their compliance by data. Ultimately, 22 women from the initial cohort included in the final analysis (Figure 1).
Table 1: Baseline characteristics of study population.
| Variable | Median (IQR)/Number (%) |
|---|---|
| Age | 58 (54.25-62) |
| BMI | 32.5 (29.0-37.6) |
| Race (Caucasian) | 11 (50%) |
| AHI | 30.8 (17.5-71.1) |
| ESS | 8 (4-12) |
| HAM-D | 7 (5-11) |
| MRS | 15 (9.5-19) |
Table 2: ESS, HAM-D, and MRS at initiation and first return visit.
| Outcome | Before | After | p-value |
|---|---|---|---|
| ESS | 8 (4-12) | 4.5 (3.0-8.5) | 0.1129 |
| HAM-D | 7 (5-11) | 5.0 (2.0-7.8) | 0.0762 |
| MRS | 15 (9.5-19.0) | 6.0 (4.0-11.8) | 0.0007 |
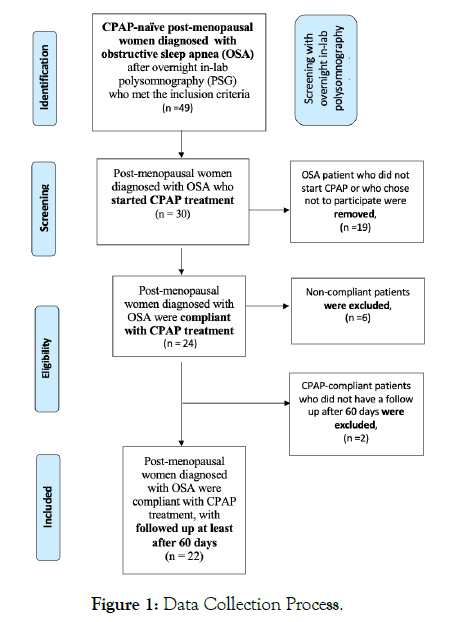
Figure 1: Data Collection Process.
There was a range of baseline apnea severity, but the average AHI was in the moderate range (median 30.8, IQR 17.5-71.1). Median subjective sleepiness was 8 (IQR 4-12) and median BMI was in the obese range at 32.5 (IQR 29.0-37.6). Hamilton Rating Scale for Depression (HAM-D) was measured at baseline for each participant. Median HAM-D was 7 (IQR 5-11) where a score of less than 7 is considered normal. Median Menopausal Rating Scale (MRS) was 15 (IQR 9.5-19.0), with MRS<4 defined as little or no menopausal symptoms, 5-8 mild; 9-15 Moderate; 16+ Severe.
Improvement in symptoms after CPAP use
Return visit occurred 158 (IQR 117-223) days after the initial visit. There was a statistically significant improvement of 6.5 points on MRS for this patient population from diagnosis to this follow up visit. There was also an improvement in HAM-D which did not reach significance at α=0.05 level, but which was significant at α=0.10 level. The associated p-values for ESS, HRSD, and MRS were p=0.1129, 0.0762, 0.0007, respectively.
While not a primary purpose of this study, further sub-analysis by baseline AHI and by race (coded as Caucasian vs non-Caucasian) revealed no effect of these variables on ESS, HAM-D, or MRS (data not shown).
Our findings demonstrate that post-menopausal women with OSA can derive substantial improvement in their menopauserelated symptoms separate from an independent effect on daytime sleepiness or depression when treated with CPAP. Although those effects did not reach significance at the α=0.05 level, there is still weak suggestion that depression may also be improved independent of improvement in sleepiness symptoms.
As life expectancy increases, women will spend more than onethird of their lives postmenopausal. Good health in old age is critical for both individual women and the whole society. Unfortunately, this transition to menopause is also associated with vasomotor symptoms, sexual dysfunction, mood changes and sleep disturbances that significantly impact quality of life. Sleep disturbances during the menopause have been ascribed to several factors, including physiological changes of aging, menopausalrelated symptoms, stress, mood symptoms (e.g. depression and anxiety), and chronic health issues. Whether sleep problems during the menopausal years are the result of interrupted sleep due to vasomotor symptoms (VMS) or whether they are separate entities is still unclear.
Post-menopausal symptoms have been traditionally treated with hormonal replacement therapy (HRT)[18], but HRT is associated with increased risk of side effects, including deep venous thrombosis and breast cancer [19]. Use of HRT has therefore declined over the years [20]. In light of this, our findings of improved postmenopausal symptoms in women with OSA treated with CPAP is of increased significance and interest, as it may offer an alternative non-HRT based therapy to improve menopausal symptoms in menopausal women with OSA.
OSA prevalence is increased in post-menopausal women, with studies suggesting a prevalence of 45.2% for mild to moderate OSA and 10.1% for severe OSA (AHI>30) [4] in contrast to the general population, where the prevalence of OSA has been reported to be 22% among men and 7% among women [21]. It is believed that the decline in sex hormones, especially estrogen, is the likely etiology [22]. OSA presents differently depending on gender, and is under recognized as women may not present with classic OSA symptoms of loud snoring, choking sensations and excessive daytime sleepiness. Women with OSA often complain of insomnia, fatigue, headache, mood disorders and lack of energy [20,23]. Therefore, it is not surprising that the ESS was within normal limits in our cohort of post-menopausal women with moderate to severe OSA. Based on our findings, we propose that clinicians and healthcare providers caring for post-menopausal women should have a higher index of suspicion and low threshold for ruling out sleep apnea in this population.
Cross-sectional and prospective studies have investigated a potential association between menopause and the risks for depressive symptoms or new onset or recurrent major depressive disorder (MDD) [24]. Data from cross-sectional studies indicate that depressive symptoms might be endorsed by up to 70% of women during perimenopause compared with around 30% in premenopausal years [25]. Studies in non-menopausal populations have shown an association between OSA and depression [26], with improvement with CPAP treatment [27]. Our data revealed a trend in improvement in depression scores (as measured by HAM-D) with CPAP treatment. Our cohort included women on antidepressants, thus emphasizing the need for ruling out OSA in this population, perhaps masquerading as depression.
Our findings demonstrate strong evidence of an improvement in menopausal symptoms in post-menopausal women with OSA treated with CPAP, independent of an effect on sleepiness or depressive symptoms. Additional clinical trials are needed to affirm these results in a larger population of menopausal women with OSA, to determine the efficacy of long-term treatment, and to garner a better understanding of the pathophysiologic mechanisms linking OSA with post-menopausal symptoms.
All authors report no conflict of interest. The authors alone are responsible for the content and writing of this paper.
Citation: Reihani A, VanHouten JP, Jain V (2020) Evaluating the effect of Obstructive Sleep Apnea (OSA) Treatment with CPAP on Menopause Rating Scale (MRS) among post-menopausal women. J Sleep Disord Ther 9:322.
Received: 30-Jul-2020 Accepted: 12-Oct-2020 Published: 19-Oct-2020 , DOI: 10.35248/2167-0277.20.9.322
Copyright: © 2020 Reihani A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.