Journal of Plant Biochemistry & Physiology
Open Access
ISSN: 2329-9029
ISSN: 2329-9029
Research Article - (2019)Volume 7, Issue 3
Superoxide dismutases (SODs) are ubiquitous metalloenzymes that constitute the first line of defense against reactive oxygen species. The role of SOD in neutralizing free radical production has already been documented. In the present investigation we have attempt to study kinetic properties of SODs in three drought sensitive and drought resistant varieties of H. vulgare. The results demonstrated that SOD displayed decreased affinities of substrate binding to their corresponding enzymes under drought conditions. The SOD isozymes profile was also analyzed and it was found that under drought conditions the only two SOD isozymes expressed as against three isozymes expressed under control. These data suggest that the decreased substrate binding affinity to SODs as well as decrease in one isozyme band of SOD under drought condition may be responsible for resistance. These findings may be used to develop drought resistance varieties of other crops.
Barley (Hordeum vulgare L.); Drought stress; SOD; Isoforms
Plants are sessile in nature and, as a result, they do not have the capability to escape from the site of unfavorable environment. As per circumstances, plants often face the challenges to grow under adverse environmental conditions such as water deficit or excess, high intense light, low or high temperature, salinity, heavy metals, UV rays, insect and pests attack, etc. These stresses wield adverse effects on plant growth and development by inducing many metabolic changes, such as the occurrence of an oxidative stress [1]. To utilize the water resources more efficiently, there is an urgent need to enhance WUE of crops, through enabling farmers to adopt need-based irrigation scheduling and efficient irrigation methods replacing calendar-based scheduling of irrigation [2]. Drought is one of the major threats to plants, as water deficit affects the plant–water relations at all levels from molecular, cellular, and organ to the whole plant [3]. Drought is a non-uniform phenomenon that influences plants contrarily.
Depending on the developmental stages, drought adversely affects physiology, morphology, growth and yield traits of wheat [4]. Barley belongs to the family of 'Poaceae', a plant commonly known as grass. The cultivation of barley in India is mainly concentrated in Uttar Pradesh, Rajasthan and Madhya Pradesh. Barley production in India is a mere 1.33 million tons out of a total grain production of 219 million tons. Although the feed portion would remain stable, the food, seed and industrial use would go up at a substantial rate. Barley is a cereal grain used in large proportions as an animal feed, while the rest is used as malt in whiskey or sugar as well as health food. Barley (Hordeum vulgare L.) is the fourth most significant cereal crop plant after wheat, maize and rice (FAO, 2003).
The recent availability of the barley genome sequence, together with various classical and large-scale approaches facilitates the identification of candidate genes involved in drought-stress adaptation [5,6]. When plants are exposed to different environmental stresses such as drought, reactive oxygen species (ROS) such as superoxide (O2•), hydrogen peroxide (H2O2), hydroxyl radicals (OH•) and singlet oxygen (1O2) are produced [7].
Plants have developed an efficient antioxidant system that can protect plants from this disaster [8]. The toxic effects of ROS are counteracted by enzymatic as well as non-enzymatic antioxidative system such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), ascorbic acid (AsA), tocopherol, glutathione and phenolic compounds, etc. Normally, each cellular compartment contains more than one enzymatic activity that detoxifies a particular ROS. The presence of these enzymes in almost all cellular compartments clears their crucial role in ROS detoxification for the survival of the plant [9,10]. Antioxidative enzymes such as superoxide dismutase (SOD), ascorbate peroxidase (APX) and catalase (CAT) play an important role against drought stress [11,12].
Superoxide dismutase (SOD, 1.15.1.1) plays a crucial role in defense against oxidative stress in all aerobic organisms, it ’ s belong to the group of metallo enzymes and catalyzes the dismutation of O2•−to O2 and H2O2. ROS production is enhanced through multiple ways. For instance, the limitation on CO2 fixation will reduce NADP+ regeneration through the Calvin cycle, hence provoking an over reduction of photosynthetic electron transport system. SOD activity increased under water stress in wheat. Antagonistic results were obtained in pea [13] in common and tepary bean [14], rice [15].
It is present in most of the subcellular compartments that generate activated oxygen. Three isozymes of SOD copper/zinc SOD (Cu/Zn-SOD), manganese SOD (Mn-SOD), and iron SOD (Fe-SOD) are reported in plants [16]. All forms of SOD are nuclear encoded and targeted to their respective subcellular compartments by an amino-terminal targeting sequence. MnSOD is localized in mitochondria, whereas Fe-SOD is localized in chloroplasts. Cu/Zn-SOD is present in three isoforms, which are found in the cytosol, chloroplast, and peroxisome and mitochondria [17].
Eukaryotic Cu/Zn-SOD is cyanide sensitive and presents as a dimer, whereas the other two (Mn-SOD and Fe-SOD) are cyanide-insensitive and may be dimmer or tetramers [18]. SOD activity has been reported to increase in plants exposed to various environmental stresses, including drought and metal toxicity [19]. Increased activity of SOD is often correlated with increased tolerance of the plant against environmental stresses. It was suggested that SOD can be used as an indirect selection criterion for screening drought-resistant plant materials. Overproduction of SOD has been reported to result in enhanced oxidative stress tolerance in plants.
Pot experiments were conducted during rabi season, at Department of Biochemistry and Biochemical Engineering, SHUATS, Allahabad. Six varieties of barley (Hordeum vulgare L.), including three drought sensitive (K-551, K-572, K-287) and three drought tolerant (K-603, K-560, K-125), were used for present investigation. The seeds were obtained from Rabi Cereal Section, Chandra Shekhar Azad University of Agriculture and Technology, Kanpur. All the seeds of different barley varieties were surface sterilized with 0.01 mM HgCl2 and inoculated in petriplates with cotton beds wet by Hoagland media. After germination, seeds were transferred in pots containing sand: soil (1:1). Seedlings were grown at 22-250 C and ~70% relative humidity under normal day light condition.
Polyethylene glycol- 6000 (PEG-6000) was used to induce drought stress. After 14 days of seedling growth, plants were divided into three groups viz. control, moderate (-1.5 bars osmotic potential) stress and severe (-3.0 bars osmotic potential) stress treatment. According to the method presented by [20] solutions with different osmotic potential were prepared for drought stress treatments. The drought stress was induced by irrigating the pot with PEG-6000 solution along with Hoagland’s solution as nutrition media. Barley seedlings were subjected to drought stress for different time intervals (24, 48 and 72 h ).
Extraction of enzyme
The leaf samples were weighed (500 mg) and ground in mortar pestle, further homogenized with 3 ml of working buffer. The homogenate was collected in a test tube and kept in the freezer for 24 h . On the next day the homogenate was filtered using muslin cloth and the supernatant collected and transferred to the eppendorf tubes which were then kept in freezer to avoid the denaturation of the enzymes.
After the extraction, enzyme was suspended in 5 ml EDTA containing potassium phosphate buffer (0.2 M, pH 7.8) and suspension was kept for 24 h at 4°C. Km and Vmax were determined by the double-reciprocal method of Lineweaver and Burk employing weighted least squares linear regression analysis [21].
SOD isoenzymes were identified in gels by a photochemical method. Gels were stained with 2.45 mM NBT for 20 minutes with constant agitation, followed by 15 minutes incubation in a solution of 28 μM riboflavin 28 mM TEMED in 50 mM potassium phosphate buffer (pH 7.8). The incubation was performed in darkness. Developing of gels was carried out with visible light during 15 min at room temperature.
In general, SOD is specific for NBT as substrate. Hence, the experiments were carried out to test the substrate specificity of SOD. The SOD was assayed at various concentrations of NBT. Lineweaver–Burk plots for NBT at 0.5-3.5 mM, which showed change in Km and Vmax values under drought stress (Table 1 and Figure 1).
| Varieties | Km (mM) | Vmax (nmoles min/mg) | ||
|---|---|---|---|---|
| Ctl | 72 h | Ctl | 72 h | |
| K-551 | 2.604 | 3.058 | 0.232 | 0.2881 |
| K-572 | 2.583 | 4.620 | 0.425 | 1.711 |
| K-287 | 1.3019 | 3.618 | 0.364 | 0.4267 |
| sK-603 | 1.904 | 2.0866 | 0.541 | 0.475 |
| K-560 | 0.999 | 2.321 | 0.321 | 0.191 |
| K-125 | 1.911 | 1.712 | 0.331 | 0.3198 |
Table 1: Km and Vmax values for SOD in control and 72 h of drought stressed plants.
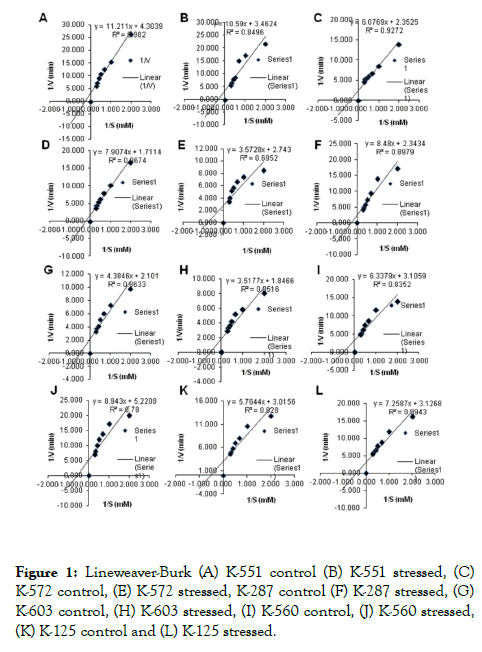
Figure 1. Lineweaver-Burk (A) K-551 control (B) K-551 stressed, (C) K-572 control, (E) K-572 stressed, K-287 control (F) K-287 stressed, (G) K-603 control, (H) K-603 stressed, (I) K-560 control, (J) K-560 stressed, (K) K-125 control and (L) K-125 stressed.
When the amount of enzyme is kept constant and substrate concentration is slowly increased then reaction velocity will increase until it reaches a maximum. A small Km indicates that the enzyme requires only a small amount of substrate to become saturated and large amount of Km indicates the need for high substrate concentration to achieve maximum velocity. Results found in this study i.e., under drought stress conditions Km value increased in comparison to control. Highest Km value was found in drought sensitive (K-551) barley variety 3.058 mM and least value (1.712 mM) was found in drought tolerant variety (K-125). Mn SOD from wheat seedlings is found to be stable over pH 7.0 -9.0 with an optimum pH of 8.0, but is sensitive to extreme pH, particularly to acidic pH. By using 1.2-2.0 mM pyrogallol and pH range from 7.5 to 10.5, Km and Vmax were determined at each pH [22].
Native polyacrylamide gel electrophoresis of SOD
A significant difference between control and severe stress (72 hours) was observed in tolerant and sensitive barley varieties for SOD, the maximum activity was obtained in tolerant group at severe stress (Figure 2).
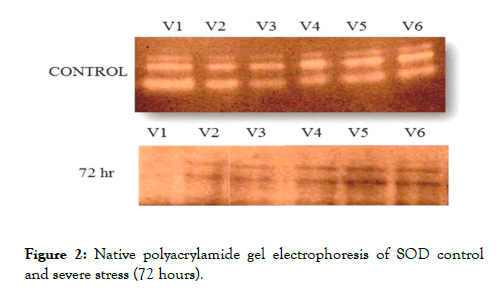
Figure 2. Native polyacrylamide gel electrophoresis of SOD control and severe stress (72 hours).
In control plants three isomers of SOD (Mn SOD, Fe SOD, Cu- Zn SOD)were found whereas in stressed plants two isomers of SOD (Mn SOD and Fe SOD) were seen and Cu-Zn SOD was not seen. Cu- Zn SOD provides less protection than Fe SODs. Because Cu-Zn SOD is located in cell wall of plants and with increase in drought stress, cell wall wizened and loose, with a decrease in cell volume. In drought tolerant barley varieties higher SOD activity was seen whereas sensitive varieties give least SOD activity under drought stress. Found that SOD activity was down regulated under severe drought stress [23]. Reported drought induced change of the isoenzyme patterns of SOD is accompanied with described change in the activity of the enzyme. The activity of SOD was high at the early stage of drought in, but SOD gene was not expressed. At the late stage of drought, SOD activity was down regulated in the droughttreated in comparison to control plants.
Superoxide dismutase is known as the first line of defense against oxidative stresses in plants and play most vital role is scavenging the reactive oxygen species produced during metabolic processes as well as under abiotic stress conditions. From the above discussion SOD displayed decreased affinities of substrate binding to their corresponding enzymes under drought conditions. These data suggest that the decreased substrate binding affinity to SOD as well as decrease in one isozyme band of SOD under drought condition may responsible for resistance. These findings may be used to develop drought resistance varieties of other crops. It is clear that the plant has more native or induced SOD activity that showed more tolerance toward abiotic stresses. Many studies have proved that higher the native SOD activity along with more number of SOD isoforms makes the plants more capable to scavenge the ROS generated during stressed condition more effectively.
Citation: Shukla N, Varma Y (2019) Enzymatic Analysis of Superoxide Dismutase (SOD) from Hordeum vulgare: its Role in Drought Stress Tolerance. J Plant Biochem Physiol. 7:238. doi: 10.35248/2329-9029.19.7.238
Received: 06-Jun-2019 Accepted: 04-Jul-2019 Published: 10-Jul-2019
Copyright: © 2019 Shukla N, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.