Reproductive System & Sexual Disorders: Current Research
Open Access
ISSN: 2161-038X
ISSN: 2161-038X
Review Article - (2021)Volume 10, Issue 4
Male Infertility is an increasing global health issue, and one of the main findings in the semen of infertile men is spermatogenesis and sperm motility deficiencies. Despite the advances in male fertility, sperm abnormalities are complex and caused by multiple endogenous and exogenous factors. The Na, K ATPase α4 isoform is testis-specific, and its catalytic function is essential to maintain sperm quality, motility, and capacity. Exogenous ouabain is known for its inhibitory actions on the Na, K-ATPase, and α4 has a high affinity for ouabain. This heightened sensitivity is the basis for numerous experiments showing that Inhibition of the α4 by ouabain leads to sperm deterioration. It is interesting to note that endogenous ouabain is a cardiotonic steroid hormone found in animals and humans during normal conditions and increases in response to high salt intake. Also, infertile men with deficient sperm motility had increased levels of endogenous ouabain in the semen. Therefore, this article emphasizes the intersectionality between the following factors: 1) high salt intake and decreased sperm motility, 2) high salt intake and increased EO levels, 3) sperm Na, K-ATPase α4 isoform high affinity for ouabain, 4) effects of high ouabain on reduced sperm quality and motility, and 5) increased EO levels in infertile men with decreased sperm motility. Based on these observations, we hypothesize that endogenous ouabain is a possible link between high salt intake and male infertility.
Cardiotonic Steroids; Endogenous Ouabain; Male Infertility; Sperm; Testis; Salt Intake; Salt Sensitivity; Na; K-ATPase; Alpha 4 Isoform.
Infertility is a global health issue affecting approximately 50-80 million people worldwide, and male factors account for 20-70% of infertility cases [1]. Most male infertility diagnoses are based on semen analysis, including sperm concentration, appearance, and motility. As such, spermatogenesis's disruption is a significant cause of infertility, with a deterioration of male sperm quality, morphology, and motility [2]. Despite the increasing knowledge regarding male infertility, idiopathic sperm abnormalities still account for about 30% of cases [3]. The etiology of male infertility is complex, and there are multiple endogenous and exogenous factors affecting semen parameters. Endogenous factors such as anatomical problems, hormonal disorders, genetic defects, and functional or immunological disturbances can explain atypical sperm production and function [4].
One of the known endogenous factors leading to male infertility in animals and humans is increased inhibition of the sodiumpotassium pump enzyme, specifically the α4 isoforms needed for proper sperm motility and fertilization. Na, K-ATPase α4 is a testis-specific isoform expressed across different animal species and humans [5]. The α4 isoform is catalytically active in sperm and has a high affinity for ouabain, a cardiac glycoside known for its differential inhibition of Na, K-ATPase [6]. Experiments using ouabain on rat sperm showed that ouabain reduced not only sperm total motility but also multiple parameters of sperm movement, such as path displacements, beat cross frequency, and linearity [7]. Furthermore, α4 deletion in male mice led to sterile animals, and their sperms were unable to fertilize eggs in vitro [8]. This study showed that sperm lacking α4 had ion imbalance, high intracellular Na+ levels, and membrane depolarization leading to infertility. In experimental studies, Yang et al., showed that intraperitoneal ouabain injection successfully induced rat asthenozoospermia (reduced sperm motility) [9]. Furthermore, Yang et al. reported significantly increased levels of the cardiotonic steroid endogenous ouabain (EO) in semen from mild and severe asthenozoospermia patients compared with normal fertility males, suggesting a role for EO in decreasing sperm motility [9].
Exogenous factors such as nutritional patterns and diets, lifestyle, and environmental factors also affect sperm. Unlike some other risk factors, intervention strategies can modify diet and lifestyle. This fact has led to numerous studies evaluating nutrients, dietary patterns, and physical activity on sperm quality [10]. Three recent cross-sectional research studies used the Dietary Approaches to Stop Hypertension (DASH) to study the association of semen quality with diet [11-13]. The DASH diet used in these studies emphasized vegetables, fruits, low-fat products, and lower sodium intake. They also limited food high in saturated fats and sugar. These studies showed that men who followed the DASH diet had better overall sperm quality, including semen volume, sperm concentration, motility, total count, and morphology. We did not find any studies evaluating the effects of high salt intake on human sperm function. Interestingly, Abdelnour et al. published a review about the impact of a high salt diet and its interference with animal male reproductive function through several different pathways leading to infertility [14]. They present extensive evidence that high salt intake has adverse effects on the male animal reproductive function, including reduction of spermatogenesis and decreased sperm function (concentration, viability, abnormalities, and motility).
This article emphasizes the intersectionality between the following five factors: 1) high salt intake and decreased sperm motility, 2) high salt intake and increased EO levels, 3) sperm Na, K-ATPase α4 isoform high affinity for ouabain, 4) effects of high ouabain on reduced sperm quality and motility, and 5) increased EO levels in males with decreased sperm motility and infertility. Based on these observations, we hypothesize that endogenous ouabain is a possible link between high salt intake and male infertility.
High Salt Intake and Endogenous Ouabain
Three decades ago, Hamlyn, Blaustein, et al., identified an ouabain-like compound in human plasma from saline expanded individuals undergoing routine plasmapheresis [15]. At the same time, Vadazs et al., reported detecting endogenous digoxin-like immunoreactivity in humans' seminal fluid[16]. Vadazs and colleagues found unbound CTS in the seminal fluid while undetected in plasma, suggesting local secretion or passive diffusion from plasma. The measurement of endogenous CTS levels such as ouabain-like and digoxin-like by immunoreactivity is still a controversial issue mainly because of the antibody's nonspecificity. Still, there is no doubt that endogenous cardiotonic steroid (CTS) such as marinobufagenin and endogenous ouabain have physiologic regulatory functions through dosedependent actions on the Na, K-ATPase [17].
During the last decade, there are relevant scientific reviews regarding Na, K-ATPase as the target for endogenous cardiotonic steroids (CTS) and the possible link between high salt diet, saltsensitivity, and organ damage. The specific identification of endogenous ouabain (EO) and marinobufagenin (MBG) in humans and their signaling pathways through the Na, K-ATPase, have been postulated to play significant roles in salt-sensitivity [18,19]. Both propose that high salt intake or inadequate sodium excretion stimulates brain EO leading to elevated MBG levels and inhibition of the vascular Na, K-ATPase resulting in vasoconstriction and saltsensitive hypertension.
The American Heart Association defines salt-sensitivity as a physiological trait by which blood pressure (BP) variations parallel salt intake changes. Liu et al., discussed the role of oxidative stress in salt sensitivity caused by endogenous CTS through the redoxsensitive Na, K-ATPase signalling [20]. Paczula et a., proposed that the increased release of endogenous ouabain in response to high salt intake activates the release of MBG, leading to vasoconstriction and organ damage [21]. Orlov et al., presented the dual role of low and high CTS doses on Na, K-ATPase activation, and inhibition, concluding that more experiments are needed to investigate endogenous CTS roles in cellular responses [17].
NA, K-Atpase ALPHA 4 ISOFORM AND OUABAIN
The Na, K-ATPase enzyme has two subunits, the alpha and beta subunits with different isoforms that tend to be more predominant according to the tissue or organ. Alpha 1 isoform is the most numerous in the human body, and the α4 isoform is only found in testis and sperm with a definite specific role in sperm function. Mature sperm cells have α1 and α4 isoforms, but the α4 has a significantly higher affinity and sensitivity to Ouabain [22]. (Table 1) summarizes several animal studies demonstrating that inhibition of Na, K-ATPase α4 with exogenous ouabain significantly decreased sperm motility [7, 9, 23]. Woo, et al. showed that the α4 isoform is expressed in the flagellum of mature sperm cells and that its inhibition eliminates sperm motility [23]. Na, K-ATPase α4 isoform's tissue specificity is shown by the results of a study using green fluorescent protein (GFP) and ATP1 4 promoter. The Atp1 α4 promoter drives GFP expression in a tissue-specific manner, being present in the testis only when mice have reached adulthood [24]. These results helped confirm that the α4 isoforms are specific and exclusively limited to the male gonad germ cells that have progressed to spermatogenesis's late stages.
| Experimental design | Ouabain effects on Sperm motility |
Reference |
|---|---|---|
| Intraperitoneal injection of saline (control), 12.5 μg/kg of ouabain x day (low dose), and 25 μg/kg x day as a high dose | With ouabain at low and high doses, the sperm motility significantly diminished compared to controls. There was no difference in motility between the high and low doses. |
Yang et al., [9] |
| Incubation of sperm in the absence or presence of 1x10-6 and 1x10-3 M ouabain |
〖10〗^(-6)M ouabain was sufficient to impair sperm motility. Further inhibition of α1 with 〖10〗^(-3) M ouabain did not result in an additional reduction of sperm motility. |
Jimenez et al., [7] |
| A. Incubation of sperm in a buffer of 1x〖10〗^(-2) M ouabain to inhibit both alpha1 and 4. B. Incubation of sperm in a buffer of 1x〖10〗^(-5) M ouabain to inhibit only alpha 4. |
A. Sperm movement was essentially abolished with little to no forward movement. B. Decreased sperm motility to the same level as inhibiting both isoforms. |
Woo et al., [23] |
Table 1: Effects of exogenous ouabain on spermatozoa from the cauda of adult rat epididymis.
To validate the importance of the Na, K-ATPase alpha 4 isoforms in male fertility, researchers have done different experiments by enhancing, deleting, and blocking the α4 Na, K-ATPase in the testes. Studies have shown that Knock-out (KO) mice that lack the Na, K-ATPase α4isoform are sterile and that this deletion hindered sperm motility and hyperactivation needed for proper fertilization [25]. McDermott et al., used the gene coding sequence for the human Na, K-ATPase α4, and transcribed the enzyme in mice sperm. Sperm carrying the human transgene exhibited enhanced total motility and increased multiple parameters, including higher sperm hyperactivity and acrosome reaction, needed to fertilize an ovum [26]. Jimenez et al., showed that sperm lacking α4 have ion imbalance, high intracellular Na+ levels, and membrane depolarization leading to infertility [8]. Syeda et al., published a comprehensive review describing the role and mechanisms of action of Na, K-ATPase α4, including using its high affinity for ouabain to design ouabain analogs for male contraception [27]. (Figure 1) illustrates the mechanism of action of Na, K-ATPase α4 to control sperm function and fertility.
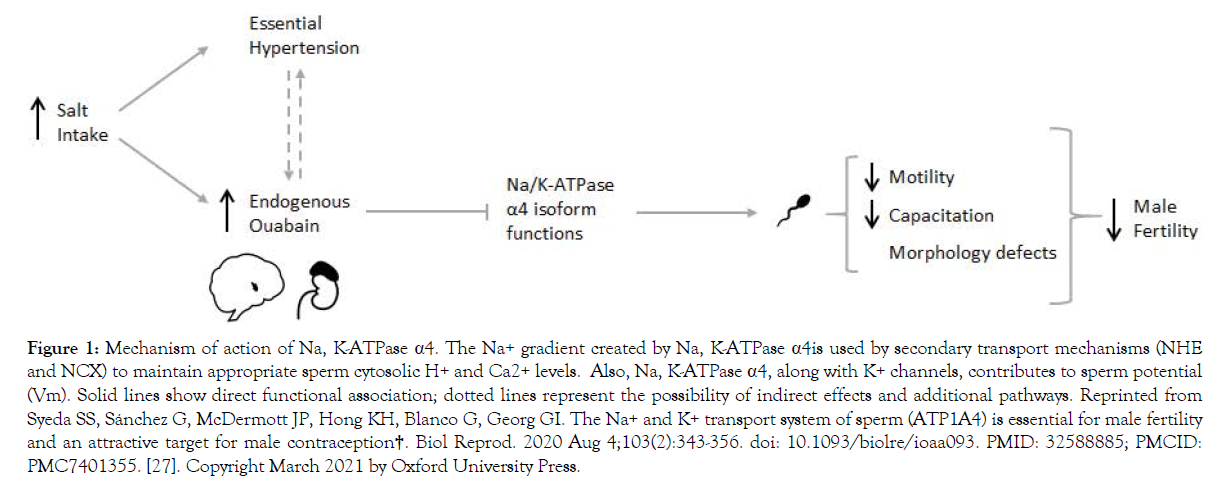
Figure 1. Mechanism of action of Na, K-ATPase α4. The Na+ gradient created by Na, K-ATPase α4is used by secondary transport mechanisms (NHE and NCX) to maintain appropriate sperm cytosolic H+ and Ca2+ levels. Also, Na, K-ATPase α4, along with K+ channels, contributes to sperm potential (Vm). Solid lines show direct functional association; dotted lines represent the possibility of indirect effects and additional pathways. Reprinted from Syeda SS, Sánchez G, McDermott JP, Hong KH, Blanco G, Georg GI. The Na+ and K+ transport system of sperm (ATP1A4) is essential for male fertility and an attractive target for male contraception†. Biol Reprod. 2020 Aug 4;103(2):343-356. doi: 10.1093/biolre/ioaa093. PMID: 32588885; PMCID: PMC7401355. [27]. Copyright March 2021 by Oxford University Press.
The mechanism of the negative impact of high salt intake on the male reproductive system merits investigation in humans because of the higher sodium content in processed and fast foods and increased male infertility across the globe. High salt intake is also known to trigger cardiotonic steroid releases, including endogenous ouabain (EO) and Marinobufagenin. Increased CTS levels are known for their inhibitory actions of the Na, KATPase activity in specific target organs. Specifically, the Na/K KATPase α4 isoform is found only in testis and sperm and has a high affinity for ouabain. Interestingly, there is an increased endogenous ouabain level in the semen of infertile men with mild and severe decreased sperm motility. (Figure 2) considers the association of high salt intake with increased circulating endogenous ouabain and the detrimental effects of high ouabain on sperm motility and fertility to hypothesize that endogenous ouabain is a possible link between high salt intake and male infertility. This hypothesis is a call for research to identify if high salt intake, salt-sensitivity, and increased levels of endogenous ouabain are underlying causes of male infertility. Other related questions are if the testis as an endocrine gland can produce cardiotonic steroids and if Marinobufagenin (MBG) has adverse effects on spermatogenesis and sperm function. Furthermore, men with other preexisting conditions related to excessive salt intake, salt sensitivity, and essential hypertension might also be associated with male infertility leading to potential health disparities.
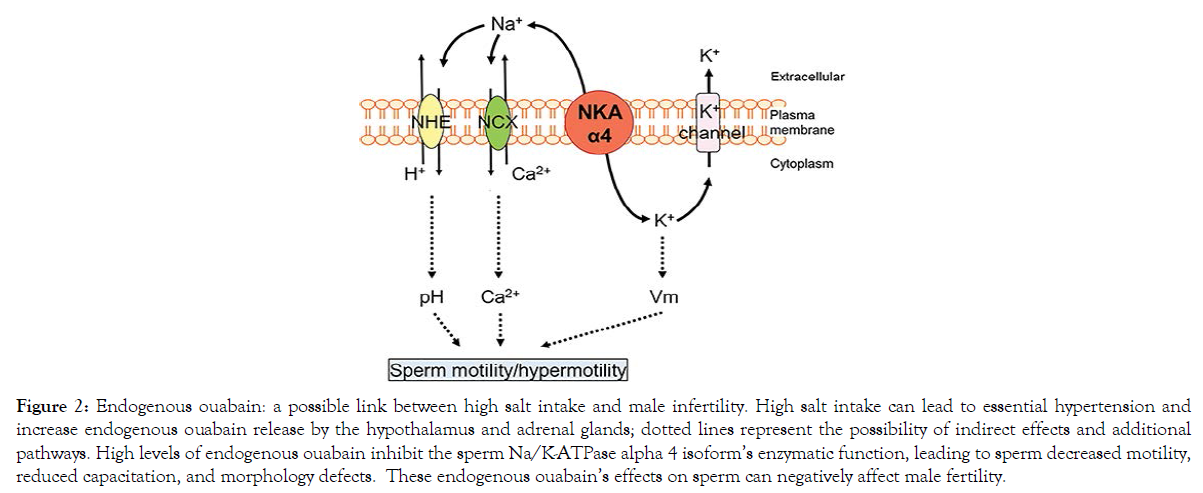
Figure 2. Endogenous ouabain: a possible link between high salt intake and male infertility. High salt intake can lead to essential hypertension and increase endogenous ouabain release by the hypothalamus and adrenal glands; dotted lines represent the possibility of indirect effects and additional pathways. High levels of endogenous ouabain inhibit the sperm Na/K-ATPase alpha 4 isoform’s enzymatic function, leading to sperm decreased motility, reduced capacitation, and morphology defects. These endogenous ouabain’s effects on sperm can negatively affect male fertility.
The following attributions are for authorship: Estapé initiated the call for conceptualizing the manuscript, final review, and editing. Lastra-Vargas is responsible for conceptualizing the hypothesis. Estape and Lastra-Vargas assume writing the original drafts. Lastra-Vargas, García-Castro, and Ocasio-Estévez took primary responsibility for literature search and revisions. Ocasio-Estévez did Figure 1, Lastra-Vargas did Table 1, and Garcia -Castro worked on the references. All authors approved the final version of the manuscript.
The authors acknowledge the outstanding contribution in the final review and suggestions of Dr. Maricarmen Colon-Diaz and Dr. Juan Carlos Jorge, experts in infertility and reproductive anatomy.
San Juan Bautista Medical School.
None to report.
Citation: Lastra-Vargas LM, García-Castro GE, Ocasio-Estévez CM, Estape ES, (2021) Endogenous Ouabain: A Possible Link between High Salt Intake and Male Infertility, Reproductive Sys Sexual Disord.10:256. doi: 10.35248/2161-038X.1000256.
Received: 05-Mar-2021 Accepted: 29-Mar-2021 Published: 05-Apr-2021 , DOI: 10.35248/2161-038X.21.10.256
Copyright: © 2021 Lastra-Vargas LM et al. this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.