Journal of Leukemia
Open Access
ISSN: 2329-6917
ISSN: 2329-6917
Research Article - (2022)Volume 10, Issue 6
An inhibitory relationship has been reported between the incidence of some parasitic infections and the development of cancer. Reported studies show that immunization with protein extracts of T. cruzi, the protozoan parasite that causes Chagas disease, prevents the appearance of tumors in 60% of mice injected with the murine lung carcinoma tumor line. The molecular bases of this process have not been elucidated, although the presence of antigens present in tumor cells and on the surface of T. cruzi suggests an anti-parasitic immune response with an effective cross-reaction against cancer cells, hence the importance to identify the antigens involved and figure out their potential as target cells in anticancer therapy. The general objective of this work is to determine the presence of antigenic proteins of T. cruzi, shared with cells in culture of ALL and NB, for this, polyclonal antibodies against T. cruzi were developed in rabbits, and the reactivity with protein extracts of cultured ALL and NB cells was determined, Likewise, the protein immunodetection of the different strains of T. cruzi was carried out with the anti-T. cruzi antibodies of the five strains. The study would also contribute to broadening the knowledge of immunological interactions between cancer and parasites in the future and therefore would broaden the panorama of new therapeutic strategies applied in oncology.
Acute lymphoblastic leukemia; Trypanosoma; Antigens; Immunotherapy; Neuroblastoma
Cancer is a generic term that designates a wide group of diseases that can affect any part of the body; A defining characteristic of cancer is the rapid multiplication of abnormal cells that spread beyond their normal limits and can invade adjacent parts of the body or spread to other organs, a process called metastasis, which is the leading cause of cancer death [1]. Although incidence rates for all cancers combined are almost twice in more developed countries respecting to less developed ones, death rates are only 8% to 15% higher in developed countries due to risk factors and screening practices, and availability of treatment [2]. In Mexico, cancer is the third leading cause of death, 14/100 Mexicans die from this disease and the life expectancy of those who suffer from it is around 63 years [3].
Acute Lymphoblastic Leukemia (ALL) is a malignant transformation and proliferation of lymphoid progenitor cells in the bone marrow, blood, and extramedullary sites. 80% occurs in children and represents a devastating disease when it occurs in adults. The incidence of ALL follows a bimodal distribution, with the first peak in childhood and the second peak around 50 years old [4]. Symptoms of ALL include enlarged lymph nodes, bruising, fever, bone pain, frequent infections, and bleeding gums. Treatment may include chemotherapy or local-release drugs that kill cancer cells. Although dose escalation strategies have led to a significant improvement in outcomes for pediatric patients, the prognosis for the elderly remains very poor [5].
Neuroblastoma is an embryonic tumor of the autonomic nervous system; it is a type of cancer that is usually found in the adrenal glands [6]. It can develop in the stomach, chest, neck, pelvis, and bones. It generally affects children under five years of age, it is the most common cancer diagnosed during the first year of life. Some of the symptoms are fatigue, loss of appetite, and fever. The clinical presentation is highly variable, from a mass that does not cause symptoms to a primary tumor that causes critical illness as a result of local invasion, widely disseminated disease or both [7].
It is historically known that parasites, both protozoa and helminths, can form long-term infections in humans whose chronic inflammation causes cancer. Some examples are Schistosoma mansoni, which causes malignant tumors [8], Plasmodium falciparum, which induces Burkitt's lymphoma [9] and Trichomonas vaginalis, which has been associated with an increase in prostate cancer [10]. The design of effective vaccines for the treatment of cancer is one of the main challenges in cancer research. Several non-pathogenic parasites such as Leishmania tarentolae, Toxoplasma gondii, and T.cruzi have been used as candidates for designing cancer vaccines [11]. There is growing experimental and clinical evidence that the immune system is actively involved in the pathogenesis and control of tumor progression. An effective antitumor response depends on the correct interaction of various components of the immune system, such as antigen-presenting cells and different subpopulations of T cells. However, malignant tumors develop numerous mechanisms to evade their recognition and elimination. The beneficial effects reported for some parasitic diseases in tumorigenesis range from the induction of apoptosis, the activation of the immune response, the avoidance of metastasis and angiogenesis, the inhibition of proliferative signals, to the regulation of inflammatory responses that promote the Cancer [12]. It has been reported for the parasitic malaria infection that inhibits cell proliferation in lung cancer [13] or by intratumoral injection of attenuated Toxoplasma gondii for cases of melanoma [14]. It was also shown that when administered to mice, three peptides from E. granulosus, these confer splenocytes with the ability to kill tumor cells. Furthermore, this cytotoxic activity was correlated with a higher number of activated NKs, which suggests that the immune response may be efficient to eliminate tumor cells in the presence of this infection [15]. Echinococcus granulosus is a cestode parasite that causes cystic echinococcosis disease. In a retrospective study a significantly lower prevalence of cancer was observed in patients with hydatid disease [16]. Antigenic similarities were found between E. granulosus and some types of tumors [17]. As well as that immunization with human Hydatid Cystic Fluid (HCF) induces antibodies against CT26 colon carcinoma cells and protects against tumor growth in mice [18].
It has also been proven that T.cruzi, the protozoan parasite that causes Chagas disease, has mediated anticancer effects, by products derived from the parasite that inhibit the growth of tumor cells, involving both the cellular and humoral components of the immune response. Therefore, it is proposed to develop polyclonal antibodies against T.cruzi in rabbits, and to determine the reactivity with NB tumor protein extracts and with protein extract of ALL cells in culture, to determine the presence of the molecules involved in this process. The aim of this study was determine the presence of antigenic proteins of T.cruzi, shared with protein extract of cells in culture of ALL and extracts protein of NB.
Parasite culture
Four different isolates of T. cruzi from infected humans: Ninoa, Silvio, Queretaro, Cuernavaca and strain CLB, were used to obtain specific antibodies of each in rabbits. Epimastigotes of 4 isolates and CLB control strain of T. cruzi were cultured, kept at 28°C in 50 mL Nunc cell culture flask of LIT medium (Liver Infusion Triptose) pH 7.2. After a period of 8 days approximately 1 × 108 parasites/mL were obtained [19].
Cellular line
ALL line SUP-B15 (RRID:CVCL_0103) was purchased and maintained in RPMI-1640 supplemented with 10% Fetal Bovine Serum (FBS) and 1 × streptomycin/penicillin antibiotics [20], and the NB line SH-SY5Y (RRID:CVCL_0019) will be kept in DMEM medium (Dulbecco's Modified Eagle's Medium, Gibco), supplemented with 10% of FBS [21]. All experiments were performed with mycoplasma-free cells.
Antigen preparation
The parasites of isolates of T. cruzi were harvested in 50 mL Falcon tubes, the medium was removed by centrifugation 2000 rpm for 20 minutes, the supernatant was removed, and two washes were carried out with saline solution at 1500 rpm for 5 minutes. Parasites were counted in a Neubauer chamber. The button formed by parasites was resuspended in 1 mL of lysis buffer with protease inhibitors and cell disruption was performed by vortexing by 5 min, follow by 3 freeze/thaw cycles [22]. Cell extract of ALL line Sup B15 was prepared from cultures in exponential growth phase, washed 2 times with PBS and subsequently incubated in a hypotonic solution (Tris 10 mM, KCl 5 mM, CaCl2 2 mM, MgCl2 1 mM supplemented with protease inhibitors at a final concentration of 500 mM EDTA, 20 mM PMSF, 10 mM leupeptin and 1 mM pepstatin,) for 30 minutes on ice. The samples were sonicated with 6 pulses of 10 seconds, the material obtained was centrifuged at 4600 rpm for 10 minutes and the supernatant was stored at -20°C until use. The protein concentration of the parasitic extracts and of the cell lines used was measured by spectrophotometry [23].
Production of antibodies against T. cruzi in New Zealand rabbits
A hyper immune anti T. cruzi serum was obtained by immunizing a male New Zealand rabbit (about 6 weeks old). Blood will be drawn before the first immunization, and this will be the pre-immune serum used as a control. It will be administered subcutaneously with 300 μg of total extract of T. cruzi epimastigotes (axenic culture) strain CL Brener, in 500 μl of complete Freund's adjuvant, 15 days later 300 μg of total extract will be administered with 500 μl of incomplete Freund's adjuvant. Later, on days 29, 30 and 31, 100 μg of total extract dissolved in saline solution will be administered intravenously, finally on day 39 venous blood will be taken from the rabbit, which will be centrifuged to separate the serum, which will be collected, aliquoted and stored at -20°C. The total IgG antibodies present in the serum was purified from the polyclonal serum generated in rabbit. For this, a protein A bound to agarose (22811, Thermo Fischer Scientific) was used.
The anti T. cruzi antibody titer was determined in the serum of the immunized rabbits by ELISA. The OD values were read at 450 nm with a reference filter of 620 nm in an ELISA reader (ELISA LKB WALLAC OLLIVETTI). Each assay plate contained negative and positive samples as controls [24].
Immunoblotting
A dot blot method was conducted with 1 mg/mL of Ag from T. cruzi isolates and immunodetected with the first antibody (inoculated rabbit serum) diluted 1:50. Protein Extract were treated with bromophenol blue and then heated to boiling for 15 minutes, thus achieving a more stable union between the SDS and the protein. Treated antigens (From T. cruzi or ALL proteins) were run in a polyacrylamide gel electrophoresis 12% for 40 min at 200 V.
Proteins were transferred to a 0.2 µM nitrocellulose membrane (Trans-Blot-Transfer Medium Bio-Rad) for 3 hours at 350 mA, 120V in transfer buffer. The membrane was blocked overnight at 4°C with 5% milk PBS, the strips of the membrane of approximately 1 cm were cut, the serum of the Anti-T. cruzi rabbits diluted 1:50 in milk PBS was added to the 1% and incubated 2 hours 37°C, positive controls were used to validate this test. After the incubation time, 3 washes with 0.1% PBS Tween were carried out, and the second anti-rabbit IgG antibody coupled to horseradish peroxidase was added in a 1:1000 dilutions with 1% milk PBS, after which time 7 washes with PBS 0.1% Tween. The strips were developed by adding the substrate 4 Chloro-Naphthol (SIGMA), the reaction was carried out at room temperature for approximately 30 min and stopped with distilled water [25,26].
Confocal microscopy
SUB15 ALL cells cultured in RPMI 1640 medium (supplemented with 10% FBS and 1X antibiotic/antimycotic) and NB cells in DMEM medium. Axenic culture of T. cruzi was used as positive control. Cells were harvested, washed with PBS. 50,000 viable cells were fixed with 2% paraformaldehyde for 15 min. After 40 min of washing with PBS, it was blocked with 2% pig serum in PBS for 1 h and the permeabilized slides were incubated with 2% pig serum and 0.5% Triton in PBS for 20 min. They were incubated for 1 h with T. cruzi antibodies 1:300 with 2% pig serum in PBS. IgG purified from unimmunized New Zealand rabbit serum was used as a negative control. Reactivity was carried out with specific anti-rabbit IgG antibodies conjugated to Alexa-Fluor 488 (Invitrogen cat no. A-11034) diluted 1:300 with 2% pig serum in PBS for 1 h. Cell nuclei were stained with 20 µg/mL of 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen cat no. D1306). The images were taken with a Leica model TCS-5P8X confocal microscope, with a 63X immersion lens, a 5X digital zoom and analyzed with the LasX software.
Determination of antibodies by ELISA
The results obtained when using the protein extract of ALL to determine the antibody titer by the ELISA method using the sera of rabbits immunized with the 5 different isolates of T. cruzi are shown in Figure 1. The ALL control had an Ab titer of 2.674 and for T. cruzi it was 1.958.
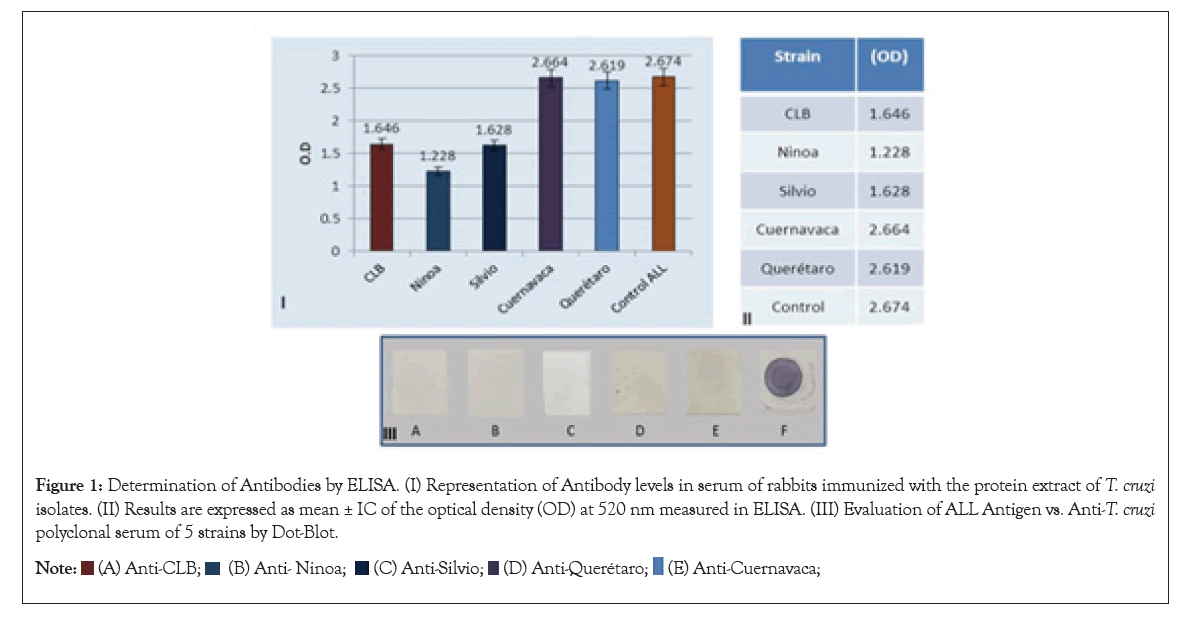
Figure 1: Determination of Antibodies by ELISA. (I) Representation of Antibody levels in serum of rabbits immunized with the protein extract of T. cruzi isolates. (II) Results are expressed as mean ± IC of the optical density (OD) at 520 nm measured in ELISA. (III) Evaluation of ALL Antigen vs. Anti-T. cruzi polyclonal serum of 5 strains by Dot-Blot. 
Immunoblot: The recognition by Western Blotting that the ALL and NB Antigen shows different specific bands for each isolate. Figure 2 shows the electrophoretic patterns of ALL and NB cells, respectively, both were run by SDS PAGE under the same conditions. The result of the immunodetection with anti T. cruzi antibodies showed the presence of proteins shared with the electrophoresed cells, in the case of ALL with the strains CLB, Cuernavaca and Querétaro while with NB, antigenic proteins were immunodetected in all strains (Figure 3).
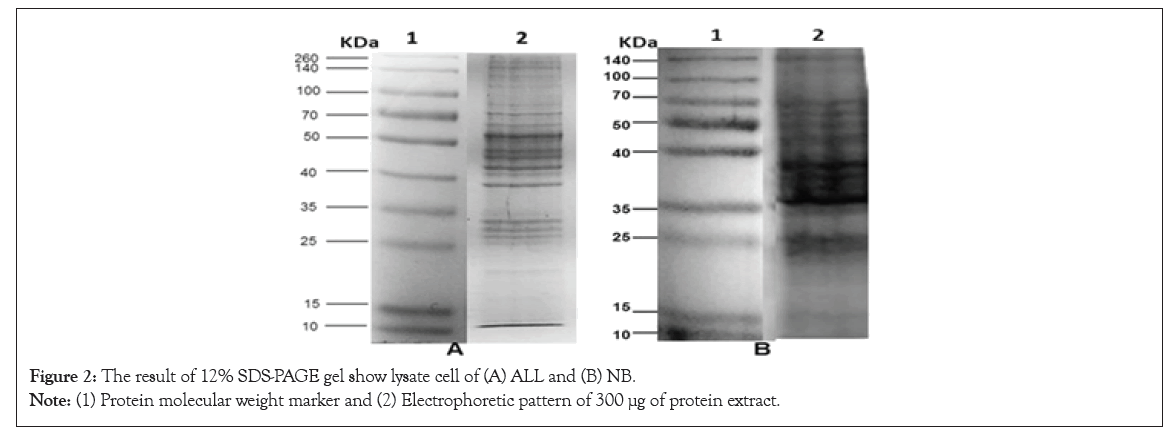
Figure 2: The result of 12% SDS-PAGE gel show lysate cell of (A) ALL and (B) NB.
Note: (1) Protein molecular weight marker and (2) Electrophoretic pattern of 300 µμg of protein extract.
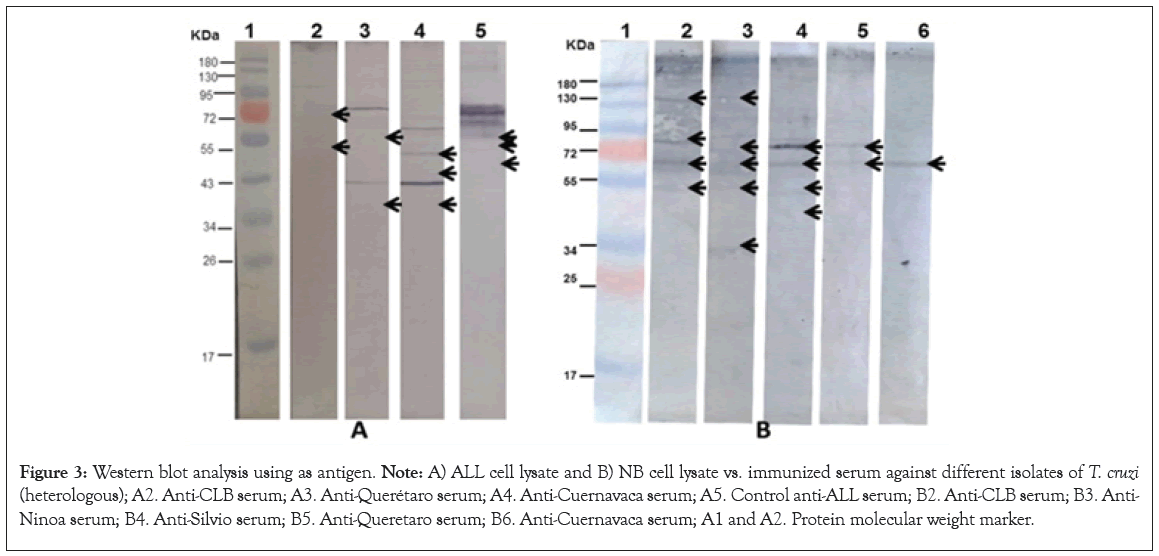
Figure 3: Western blot analysis using as antigen. Note: A) ALL cell lysate and B) NB cell lysate vs. immunized serum against different isolates of T. cruzi (heterologous); A2. Anti-CLB serum; A3. Anti-Querétaro serum; A4. Anti-Cuernavaca serum; A5. Control anti-ALL serum; B2. Anti-CLB serum; B3. Anti-Ninoa serum; B4. Anti-Silvio serum; B5. Anti-Queretaro serum; B6. Anti-Cuernavaca serum; A1 and A2. Protein molecular weight marker.
The homologous immunodetection of Ab: was made by western blot using the protein extract of T. cruzi strain as antigen and reveling with ab anti- T. cruzi of each of five strains of T. cruzi (Figure 4).
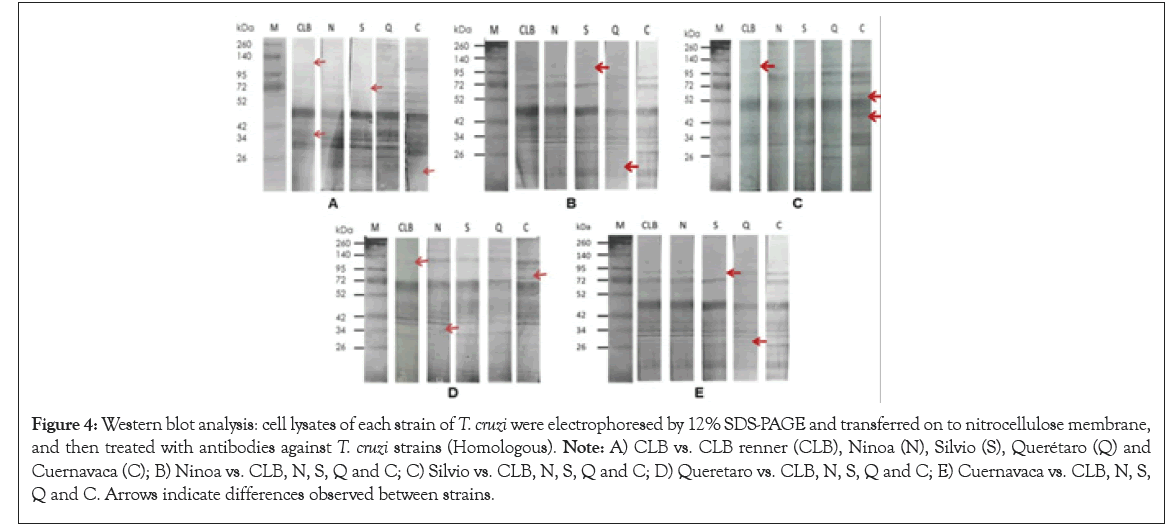
Figure 4: Western blot analysis: cell lysates of each strain of T. cruzi were electrophoresed by 12% SDS-PAGE and transferred on to nitrocellulose membrane, and then treated with antibodies against T. cruzi strains (Homologous). Note: A) CLB vs. CLB renner (CLB), Ninoa (N), Silvio (S), Querétaro (Q) and Cuernavaca (C); B) Ninoa vs. CLB, N, S, Q and C; C) Silvio vs. CLB, N, S, Q and C; D) Queretaro vs. CLB, N, S, Q and C; E) Cuernavaca vs. CLB, N, S, Q and C. Arrows indicate differences observed between strains.
Confocal microscopy: Figure 5 shows the result of the confocal microscopy assay with ALL and NB cells against anti T. cruzi antibodies, using DAPI and Alexa 488 as flu orogenic markers. Rabbit pre-immune serum was used as a negative control and T. cruzi cells were used to show the specificity of the antibodies.
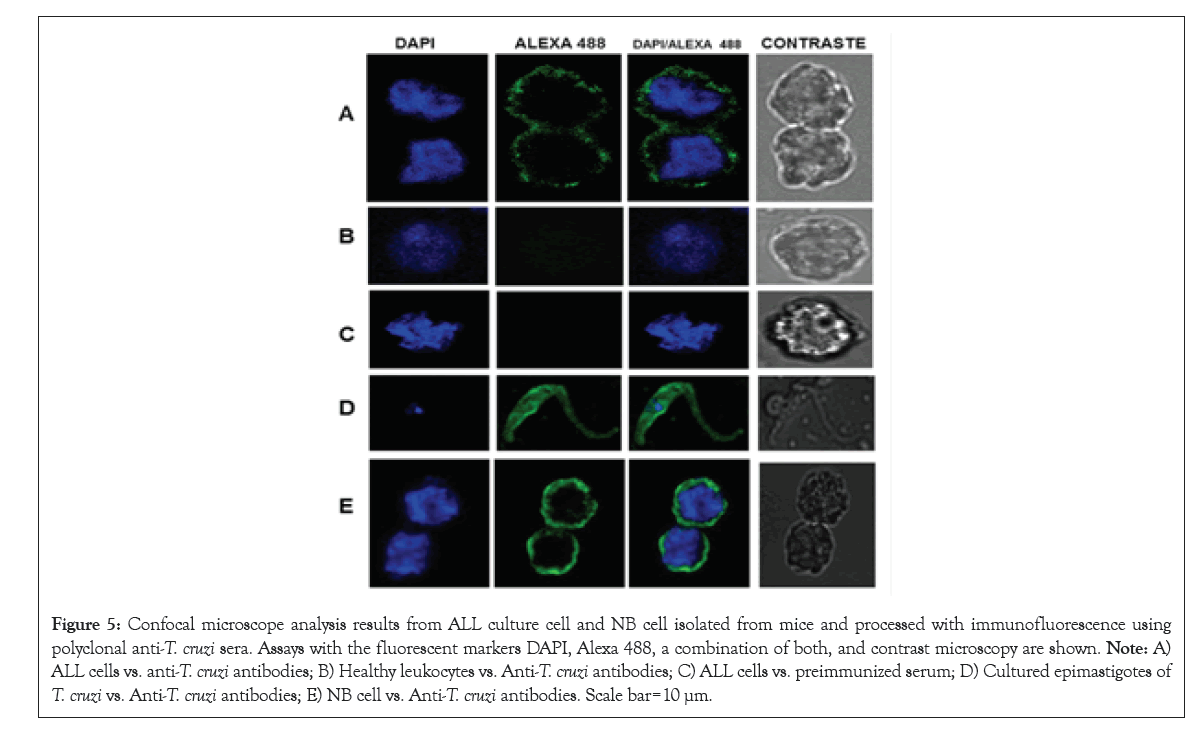
Figure 5: Confocal microscope analysis results from ALL culture cell and NB cell isolated from mice and processed with immunofluorescence using polyclonal anti-T. cruzi sera. Assays with the fluorescent markers DAPI, Alexa 488, a combination of both, and contrast microscopy are shown. Note: A) ALL cells vs. anti-T. cruzi antibodies; B) Healthy leukocytes vs. Anti-T. cruzi antibodies; C) ALL cells vs. preimmunized serum; D) Cultured epimastigotes of T. cruzi vs. Anti-T. cruzi antibodies; E) NB cell vs. Anti-T. cruzi antibodies. Scale bar=10 µm.
T. cruzi, the protozoan parasite that causes Chagas disease, shows anticancer effects, by the presence of proteins that inhibit tumor cell growth [27]. There are scientific reports that introduce various molecular targets having the antitumor ability. However, it has not been clearly elucidated which is the molecule with anticancer activity, nor the process that is carried out. The reported studies have been in cell cultures and experimental animals and suggest following facts: T. cruzi has antitumor effects by inducing host immunity against tumor. T. cruzi expresses a calreticulin that can directly interact with endothelial cells and inhibit their proliferation, migration and capillary morphogenesis as well as inhibit tumor cell [28,29].
The immunization of laboratory animals with total extracts of T. cruzi strains, allowed obtaining sera with a high antibody titer, obtained by ELISA (greater than 1.6), however it is observed that the immunogenicity is different between them, since the Silvio and Ninoa isolates present a lower test titer (Figure 1). A dot blot was performed to ensure that there was an immunogenic reaction between the immunized serum and the protein extract of the ALL cells in culture, and although the detection signal was weak, it allowed ensuring the presence of polyclonal antibodies, which could be used in the western blot method.
Figure 2 shows the different electrophoretic patterns of a cell extract of ALL and NB, showing the presence of proteins that vary in size and pH, and are the basis for later performance of western blot. The results of the western blot Figure 3 shows that T. cruzi do share antigenic molecules with the ALL cells in culture and the NB extract developed in a mouse, which were recognized by specific antibodies present in serum. In the case of ALL, the recognition was with antibodies from CLB, Queretaro and Cuernavaca strains, where the recognition of fragments of 95, 75, 70, 55, 50 and 43 KDa is seen. With the antibodies against Silvio and Ninoa strains they did not show recognition, so they are not included in the figure. Regarding NB, recognition is observed with the immunized sera of the five strains, observing bands of 140, 90, 85, 80, 70 and 55 KDa, some of more intensity than others and that are recognized by the different isolates. In this case, a reaction was observed with the five strains, unlike ALL, which indicates that the genetic plasticity of T. cruzi also influences the recognition of shared antigenic proteins.
This plasticity is observed in Figure 4, where immunized serum against T. cruzi isolates recognized antigens in homologous protein extracts, and among them the presence of different antigens is observed, which may be the origin of the differences between strains when recognizing antigens of ALL and NB. Reported research indicates that the antitumor capacity of T. cruzi is influenced by the Discrete Typing Unit (DTU) to which the strain corresponds [30].
The heterologous immunodetection show that there are shared antigens between the extracts of ALL and NB cells and T. cruzi cells and in the other hand, the homologous immunodetection shows that the protein plasticity between strains of T. cruzi is remarkably high, since the antibodies obtained from each one by immunization in rabbits shows significant differences. Although among factors, the presence of T. cruzi and other infectious organisms have been reported to be involved in carcinogenesis, some of them with antitumor capacity have also been reported [31]. This study is a contribution to figure out the proteins involved in the mechanism that causes some T. cruzi proteins to induce a decrease in some oncological processes, since still the specific mechanisms of action are not totally clear.
Confocal microscopy assay showed the presence of antigenic proteins by immunodetection in ALL and NB cells with antibodies against T. cruzi. Recognition is more intense in NB cells than in ALL cells. These results evidence the shared proteins of T. cruzi with ALL and NB cells. Therefore, it shows the need for further research to determine which are the most important and how they are involved in the anticancer capacity of Trypanosoma [32,33].
This study suggests that infection by T. cruzi generates a powerful immune response capable of enhancing and directing an efficient immune response against oncological cells. T. cruzi antigens have common epitopes with mammalian mucins and with glycoproteins that share sialyl-Tn-like structures (specific human structures associated with cancer. We think all researchers are necessary to design studies of the antitumor action of T. cruzi and their products to the discovery and use of new molecules that may be proposed as therapeutic alternatives in treatment of cancer.
The authors manifested no potential conflicts of interest.
[Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar],
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
Citation: García LE, Guerrero MS, Pilar del MCV, Acosta VAM, Campos AC, Cardoso EJ (2022) Trypanosoma cruzi Antigenic Proteins: Shared with Acute Lymphoblastic Leukemia and Neuroblastoma. J Leuk. 10:307.
Received: 19-Jul-2022, Manuscript No. JLU-22-18494; Editor assigned: 22-Jul-2022, Pre QC No. JLU-22-18494; Reviewed: 08-Aug-2022, QC No. JLU-22-18494; Revised: 16-Aug-2022, Manuscript No. JLU-22-18494; Published: 24-Aug-2022 , DOI: 10.35248/2329-6917.22.10.307
Copyright: © García LE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.