Journal of Leukemia
Open Access
ISSN: 2329-6917
ISSN: 2329-6917
Short Communication - (2022)Volume 10, Issue 3
The P47phox cytosolic protein, also known as neutrophil cytosolic protein 1, is historically regarded and significant as a central component of the NADPH oxidase complex responsible for oxidative metabolism in mature myelomonocytic hematopoietic cells such as neutrophils. It is a subunit of the neutrophil oxidase complex and essential for its activation. Its mutation is associated with various immune and hematological pathologies such as Sjorgen’s Syndrome, rheumatoid arthritis and lupus erythematosus, possibly hinting at broader functions. This 47kD protein has a number of protein interfaces/functional domains and becomes heavily phosphorylated [1]. Studies of retinoic acid-induced differentiation of model GM (Granulocyte-Monocyte)-precursor cells into neutrophils have indicated that retinoic acid rapidly induces expression of p47phox within hours of retinoic acid treatment-which is very early in the process of induced differentiation, a process that unfolds over about 72 hours in these cells [2]. This induced differentiation depends on activation of a cytosolic signaling complex, a signalosome, which contains a number of MAPK pathway related signaling molecules and is dependent on various scaffolding molecules that become phosphorylated to propel differentiation to neutrophils [3]. Significantly p47phox has protein interfaces such as SH3 that are known to collaborate with such signaling molecules as constitute this signaling machine seminal to differentiation. Hence it may provide a needed scaffold in forming this large macromolecular signalosome complex. There is thus motivation because of its early, rapidly induced expression, its phosphorylation, and its potential interfaces for signalosome proteins that drive differentiation to suspect that it functions not only in the mature neutrophil oxidative metabolism to produce superoxide, but to drive the process of retinoic acid-induced differentiation of immature precursor cells to mature neutrophils.
We explored this conjecture by making a p47phox knockout and determining if it was still capable of retinoic acid-induced differentiation. The simple anticipation based on the historically dominant paradigm is that loss of p47phox would cripple differentiation along the neutrophil lineage and in particular prevent inducible oxidative metabolism superoxide production. The results were highly unexpected and contrary to anticipation. Not only did the immature cells with the knockout differentiate, but the differentiated cells still retained the capability to undergo inducible oxidative metabolism evidenced by the production of superoxide despite the absence of p47phox.
GM lineage uncommitted precursor cells, the HL-60 human leukemia cell line, were subject to CRISPR/Cas9 knockout targeting p47phox to create p47KO stable transfectants. The parental wild type (wt) cells were used as a control to compare to for the effect of the knockout. Retinoic acid induces the differentiation of the wt cells along the myeloid (granulocytic) lineage resulting in the progressive expression of a series of cell surface differentiation markers, CD38 and CD11b, and then functional differentiation marked by inducible oxidative metabolism and G1/0 cell cycle arrest in mature cells. The wt and p47KO cells were untreated or RA-treated (10-6M) for 72 hours. And then their expression of CD38, CD11b, inducible ROS (Reactive Oxygen Species, Superoxide), and G1/0 DNA enrichment betraying growth arrest were measured by flow cytometry. The experimental procedures were all performed as previously described (Figures 1-5) [4,5].
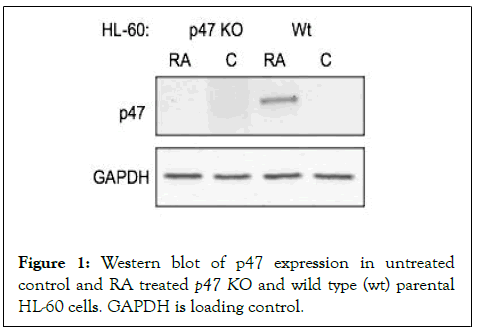
Figure 1: Western blot of p47 expression in untreated control and RA treated p47 KO and wild type (wt) parental HL-60 cells. GAPDH is loading control.
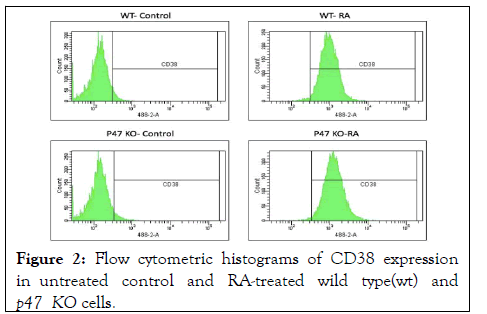
Figure 2: Flow cytometric histograms of CD38 expression in untreated control and RA-treated wild type(wt) and p47 KO cells.
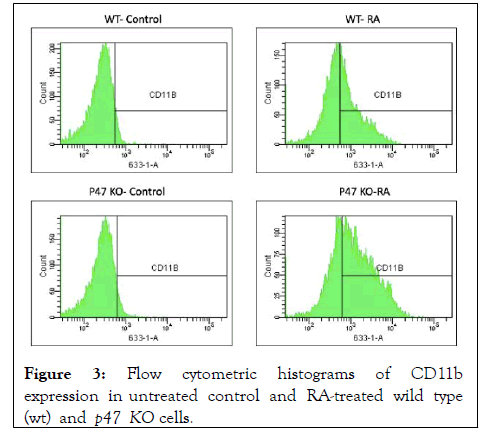
Figure 3: Flow cytometric histograms of CD11b expression in untreated control and RA-treated wild type (wt) and p47 KO cells.
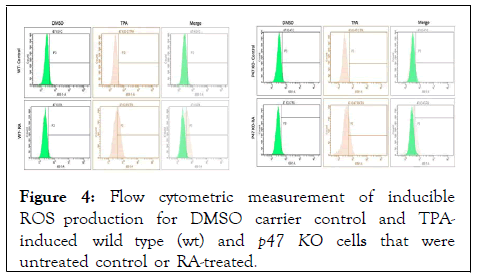
Figure 4: Flow cytometric measurement of inducible ROS production for DMSO carrier control and TPAinduced wild type (wt) and p47 KO cells that were untreated control or RA-treated.
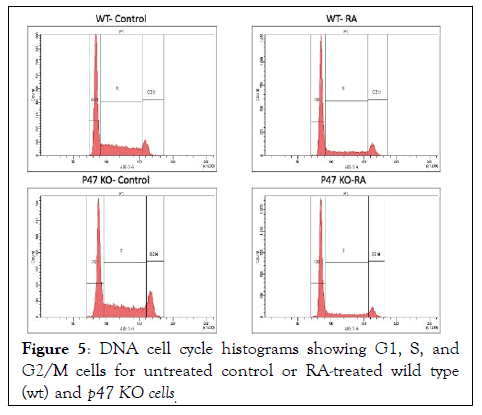
Figure 5: DNA cell cycle histograms showing G1, S, and G2/M cells for untreated control or RA-treated wild type (wt) and p47 KO cells.
The p47KO cells do not express p47phox after RA treatment (72 hrs.) unlike wt cells where RA induces p47phox expression. The p47phox gene was disrupted using CRISPR/Cas9 targeting the first exon. Western blotting of untreated and RA-treated wt and p47KO showed no detectable RA-induced p47phox expression in the knockout compared to the wt cells where prominent expression was induced.
RA-induced expression of CD38 was unaffected by loss of p47phox. CD38 is an ectoenzyme receptor that is an early marker as RA induces differentiation toward mature myelomonocytic cells. Flow cytometric analysis of CD38 immunofluorescence of untreated versus RA-treated wt and p47KO cells showed that RA induced CD38 expression in the knockout, as well as the wt cells. Loss of p47phox ergo did not block this early step of differentiation. (All histograms represent a sample of n>10,000 events).
RA-induced expression of CD11b was also unaffected by loss of p47phox. CD11b is an integrin receptor subunit that is a later marker of RA-induced differentiation toward mature cells. Flow cytometric analysis of CD11b immunofluorescence of untreated versus RA-treated wt and p47KO cells showed that RA induced CD11b expression in both the knockout, as well as the wt cells.
RA-induced expression of the functional differentiation marker, inducible oxidative metabolism, characterizing mature cells was also not lost in the absence of p47phox. Respiratory burst was analyzed by measuring inducible ROS production by flow cytometry using the 2',7'-dichlorofluorescein (DCF) assay. TPAwith a DMSO carrier-was used to stimulate ROS production. RA-treated wt and p47phoxKO cells both exhibited TPA-induced ROS (Pink histogram in TPA column showing increase due to RA for wt and for p47KO cells).
RA-induced G1/0 cell cycle arrest characteristic of mature cells was unaffected by loss of p47phox. Flow cytometry was used to analyze DNA histograms after DNA staining with propidium iodide. G1/0 arrest is betrayed by enrichment of the relative number of G1-DNA cells. Both wt and p47KO cells showed RAinduced accumulation of cells in G1/0.
Hence, even though expression of p47phox is induced early and the protein has functional domains that could bind signaling molecules known to propel RA-induced differentiation, suggesting a role as a scaffolding molecule for the large signaling machine known to enable RA to induce differentiation, its loss does not prevent differentiation; and even more surprisingly does not prevent inducible ROS production that is a functional marker for mature granulocytic series cells. The latter is particularly very surprising since p47phox is a component of the NADPH oxidase complex.
We are grateful for support from Dr. John Babish, Paracelsian and Cornell Stem Cell Prog.
[CrossRef], [Google Scholar], [PubMed]
[Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
Citation: Kazim N, Yen A (2022) P47phox is not necessary for Differentiation to Neutrophil Lineage and Oxidative Metabolism. J Leuk. 10:292.
Received: 14-Mar-2022, Manuscript No. JLU-22-16263; Editor assigned: 17-Mar-2022, Pre QC No. JLU-22-16263 (PQ); Reviewed: 31-Mar-2022, QC No. JLU-22-16263; Revised: 04-Apr-2022, Manuscript No. JLU-22-16263 (R); Published: 11-Apr-2022 , DOI: 10.35248/2329-6917.22.10.292
Copyright: © 2022 Kazim N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.