Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Review Article - (2021)Volume 10, Issue 5
Since the prehistoric period medicinal plants have been manoeuvred for disease cure. Cancer is a global problem which is widely affecting the world. Plant derived natural products have gained much importance in the field of cancer chemotherapy during the recent past and the search for new drugs for cancer treatment still continues. Andrographis paniculata (Brum. F) Nees, a highly recognized plant of traditional Chinese and Indian healthcare system has gained the consideration of researchers for cancer cure. A. paniculata has multiple possible pharmacological actions. This review reports detailed information on major phytochemicals, traditional applications, pharmacology, and methodology of phytochemical purification from A. paniculata. Its major active phytochemical Andrographolide have shown significant anticancer properties. These characteristics of A. paniculata make it a strong candidate as a prospective plant derived anticancer drug.
Medicinal plants; Anti-cancer; Phytochemicals; Andrographis paniculata; Andrographolide; Immunomodulatory
Ever since ancient times, people have been studying nature for disease cure [1]. Plants having medicinal properties have been used around the world for thousands of years [2]. As claimed by World Health Organization (WHO), 65%-80% community of developing countries is currently using medicinal plants as remedy against different diseases [3]. At least 121 chemical substances with known structures have been extracted from plants and are being used as medicines worldwide [4]. Medicinal plants and aromatic plants provide a variety of compounds from raw material to processed and packaged products such as medicines, herbs, teas, cosmetics, dietary supplements etc. In China, approximately 80% of its medicinal plant products are harvested from wild sources and approximately 32,600 tons raw material is exported from China. In Asia-Pacific, more than 8000 types of plants are officially recognized for their pharmaceutical attributes. More than 4000 types of medicinal plants are discovered in Mainland China [5]. To cure the body, in case of alternative therapy different components of the medicinal plants for example, leaves, roots, flowers are consumed [6]. As claimed by WHO, approximately 80% of human population used phytotherapy worldwide (WebMD) [7].
Cancer: A global thread
Any disease distinguished by the growth of anomalous cells which split wildly and can easily penetrate and demolish normal cells of the body leading to despondency and mortality is called Cancer (Mayoclinic) [8]. After cardiovascular diseases, amid non-infectious diseases, cancer is the 2nd prime source of deaths [9]. Every year millions of citizens are diagnosed with cancer and greater than fifty percent of the patients pass away because of cancer [10]. In 2018, 18.1 million new cancer cases and 9.5 million causalities have been reported across the globe. In 2019, 1.7 million latest victims and 0.6 million cancer casualties were expected in the United States. The range of occurrence of cancer was steady in women and fall of roughly 2% in men over the last ten years (2006-2015). The mortality rate reduced by 1.4% in women and 1.8% in men per annum (2007-2016) [11]. It is reported that the occurrence of cancer is anticipated to 29.5 million and cancer related casualties will reach 16.4 million by 2040. In general, the prevalence of cancer is excessive in countries with high up life’s duration, literacy rate and standard of living. But for particular types of cancer such as cervical cancer, the circumstances are absolutely dissimilar. The occurrence is on high where these actions are lower (NIH) [12]. Various types of cancer explained by Farley et al. [13] have been described in Table 1.
| Brain metastasis | Lung carcinoma | Pancreatic carcinoma |
| Breast carcinoma | Leukemia | Prostate cancer |
| Colon cancer | Laryngeal carcinoma | Urinary bladder cancer |
| Cancer of cervix | Lymphoma | Malignant ascites |
| Fibrosarcoma | Malignant cancer of melanocytes | Skin cancer |
| Gastric cancer | Nephrocarcinoma | Thyroid cancer |
| Lymphosarcoma | Ovarian carcinoma | Uterine cancer |
| Liver cancer | Oral cancer |
Table 1: Different forms of cancer.
Anti-cancer treatment
As the cancer grows, cancer tumors become highly heterogenous and form a mix population of cells which are characterized by different molecular features and diversified repose to treatments. Normally, cancer is managed as a universal and un-distinguishable ailment and cancerous growths are examined as a complete culture of cells. Thus, for the development of detailed and systematic remedies, extensive interpretation of these difficult circumstances is very important [14].
There are many cancer treatment strategies; however, treatment option should be selected by identifying the type and stage of cancer. Anticancer treatment choices involve incision, chemotherapeutic agents, and radiology. These three are considered as the mainstream of oncological treatment but risks of injury and toxicity to normal cell are associated with them [15]. Other cancer treatments include transplantation of bone marrow, hormone replacement therapy, targeted dug therapy, Cryoablation therapy, microwave ablation as well as other clinical investigations and studies. Many other methods of cancer treatment are still being investigated and clinically tested. These methods include photochemotherapy or phototherapy, use of ultrasound and sonosensitizers, T-cell biological response modifier therapy and oncolytic virus therapy. These management choices are anticipated to impart superior and best results because of their efficient processes for the elimination of tumor [16]. There are certain side effects associated with these treatment options such as toxicity, non-specificity, fast clearance, and restriction in metastasis. In case of radiation therapy there are also certain risks and side effects such as erythema, atrophy, dermatitis, hair loss, radiation cystitis, and bowel discomfort etc. [17].
Anti-cancer drugs
Chemotherapy is the most capable method for treating cancer. More up to date drugs are being developed that attack cancer cells completely in different ways. These drugs provide targeted therapies and provide less harm to typical cells [18]. In the past decade, 26 latest antineoplastic medicines had been approved by Food and Drug Administration Authority (FDA) [19] (Figure 1 and Table 1).
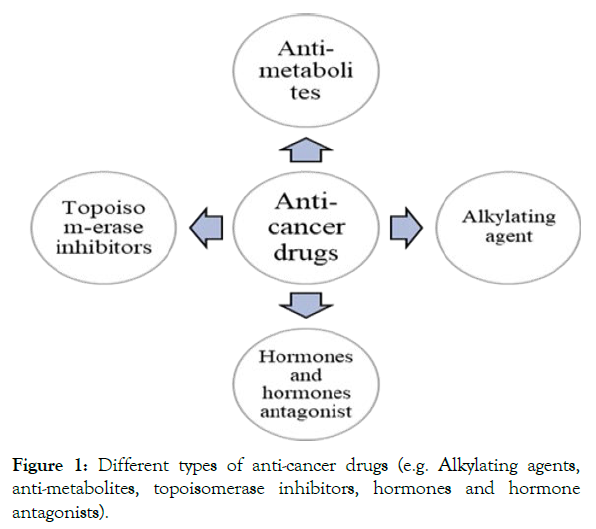
Figure 1: Different types of anti-cancer drugs (e.g. Alkylating agents, anti-metabolites, topoisomerase inhibitors, hormones and hormone antagonists).
Alkylating agents: For over six decades, alkylating agents are used for curing cancer related issues; however their collection proceeds to develop. These agents act amid all the stages of the cell cycle, specifically on DNA, crosslinking the N-7-guanine buildups, causing DNA strand breaks, driving to anomalous base blending, restraint of cell division and in the long run coming about in cell passing. Their systemic toxicity and drug resistance are major obstacles in limiting their clinical efficacy [20].
Antimetabolites: These were first found to have clinical antitumor activity almost many years ago. In the following 10 years methotrexate, 5-fluorouracil and 6-mercaptopurine found their way in clinical practice [21]. Antimetabolites are utilized broadly in chemotherapy combinations for treatment of different leukemias and strong tumors. These combinations may increase the induction of apoptosis [22].
Topoisomerase inhibitors: Topoisomerase inhibitors meddled with topoisomerase enzymes (topoisomerase I and II), can control the changes related to DNA structure. These block the ligation step of cell cycle, as a result of which DNA single and double strands break, leading to apoptotic cell death. Topoisomerase I inhibitors are irinotecan, topotecan, and camptothecin and topoisomerase II inhibitors are etoposide, doxorubicin, and epirubicin [23].
Hormones and hormone antagonists: Hormone treatment is nontoxic and effective for estrogen and progesterone receptor- positive breast cancer and prostate cancer. To reduce the size of primary cancer before radical surgery or radiotherapy and to minimize the risks of reappearance hormone therapy can be used [24].
Limitations of chemotherapy
Chemotherapy is known for critical harmful impacts and adverse effects, with most strategies focused on non-specifically isolating the cells quickly whether they are tumors or not. These agents damage normal as well as cancer cell which may lead to serious side effects and sometimes even cause the death of the patient [25]. Moreover, poor pharmacokinetics of medicines used for treatment of cancer emerging from poor solubility, stability, and metabolism. These results in inefficacy, toxicity and limited bio-distribution [26].
Cancer therapy via phytochemicals
The side effects of the chemotherapeutic agents in some cases is a critical issue in treatment of cancer utilizing allopathy and set up medications. Different treatment options are proposed for the treatment of cancer, numerous of which utilize plant-derived products. For centuries, plants have been recognized for their anticancer properties. Approximately 35,000 plants having potential anticancer activities has screened by The National Cancer Institute (NCI). Out of which, almost 3,000 plants show established anticancer activity [27]. Approximately 60% of the drugs utilized for cancer treatment right now are isolated from natural products making the plant kingdom a noteworthy source of anticancer drugs [28].
Many studies have focused on identifying the anti-carcinogenic properties of the plants and development of new chemo-protective agents. There are number of plants which are still being actively researched and have shown promising anticancer properties. So many other latest chemo-protective agents had been recognized and categorized centered upon their ability to modify more than one particular biological conditions. The scientific breakthrough of these agents and their underlying mechanism of action can help to contribute to the discovery of new alternative and complementary methods for cancer treatment. A variety of anticancer compounds obtained from plants species such as vinblastine, camptotecan and Taxol which had been effective against various cancers and are remarkable in this regard [29]. Phytochemicals and their derived metabolites present in leaf, bark, stem, root perform several pharmacological effects in human system. Alkaloids, flavonoids, tannins, phenolics, glycosides, gums, resins and oils are such useful fractions [30]. These compositions perform integral responsibilities in either inhibiting the tumor cell activating proteins, enzymes, or directing pathways or by triggering and DNA overhaul process [31].
Andrographis paniculata vernacular names
Andrographis paniculata (Brum. F) Nees, frequently known as the king of bitters, is an herbaceous plant belonging to family Acanthaceae. In India, it was named as “Kalmegh” while it was known as “Chuan-Xin-Lian” in China. In Thailand it is named as “Fah Tha Lai” whereas it is pronounced as “Hempedu bemi” in Malaysia. It is called “Senshiren” in Japan, and in Scandinavian nations it is termed “green chiretta” [32]. It is broadly practiced in traditional medicine systems of India, Pakistan, China, Hong Kong, Malaysia, the Philippines, Indonesia and Thailand [33].
Habitat
Andrographis paniculata is widespread across the arctic and subarctic zones of Southeast Asia. Across India, Pakistan, Indonesia it prevails amply but in mainland China, Thailand, east and west indies and Mauritius Andrographis paniculata cultivated on large scale [34]. The image of A. paniculata shown in Figure 2.
Figure 2: Andrographis paniculata.
Botanical description of plants
Andrographis paniculata is annual, divaricate, straight herbaceous plant which is produced in fences all between the horizontal surfaces, slope slants, squander grounds, farms, damp environment, sea shores, and road sides. It can also cultivate in gardens. Forests, moist shady places are suitable for its growth [6]. The morphological physiological traits of A. paniculata are presented in Table 2 [35] and Figure 3 [36].
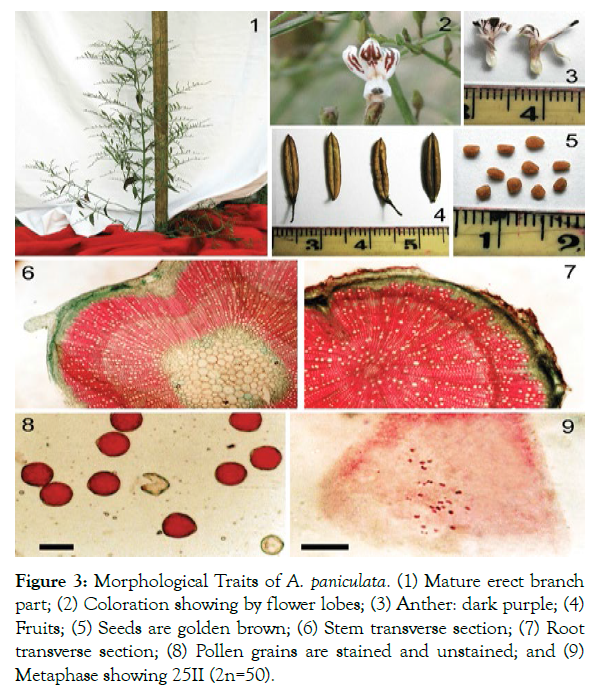
Figure 3: Morphological Traits of A. paniculata. (1) Mature erect branch part; (2) Coloration showing by flower lobes; (3) Anther: dark purple; (4) Fruits; (5) Seeds are golden brown; (6) Stem transverse section; (7) Root transverse section; (8) Pollen grains are stained and unstained; and (9) Metaphase showing 25II (2n=50).
| Attributes | Values |
|---|---|
| Height of plant | 30-110 cm |
| Color of stem | Dark green |
| Length of stem | 30-100 cm |
| Diameter of stem | 2-6 mm |
| Shape of stem | Quadrilateral |
| Pattern of leaves | Hairless |
| Length of leaves | 2-12 cm |
| Width of leaves | 1- 3 cm |
| Arrangement of leaves | Unsubdivided |
| Leaves appearance | Feather like, delicate peaks, whole periphery |
| Color and arrangement of flowers | Purple-rose dots above petals with white color |
| Area of flowers | Small, subsidiary |
| Shape of seeds | Linear, oblong capsules |
| Size of seeds | 1.9 × 0.3 cm |
| Color of seeds | Brown yellowish |
| Shape of seeds | Square but with rounded corners, many |
| Flowering and fruiting time duration | December-April |
Table 2: Physiological traits of Andrographis paniculata.
Ethnobotanical uses
The comprehensive standard uses of A. paniculata are given in Table 3.
| Countries/traditional medicine system (TMS) | Medicinal uses | References |
|---|---|---|
| Traditional Chinese medicine | Inflammation, burn, common cold, eczema, fever, respiratory infections, pelvic inflammation, snake bite, etc. | (Akbar, 2011) |
| Traditional Indian medicine | Enteritis, dysentery, diabetes, herpes, skin infections, peptic ulcer | (Jarukamjorn & Nemoto, 2008) |
| Ayurvedic | Fever, vitiligo, liver disease, torpid liver | (Boopathi, 2000) |
| Malaysia | Diabetes, hypertension | (Borhanuddin et al., 1994) |
| Japan | Fever, common cold | (Borhanuddin et al., 1994) |
Table 3: Medicinal uses of Andrographis paniculata.
Major phytoconstituents of A. paniculata
Diterpenoids, flavonoids, and polyphenols are the pre-eminent phytochemicals produced buy A. paniculata [37]. Andrographolide is the vital diterpenoid which constitute about 4% of entire dried plant, 0.8% to 1.2% of stem and 0.5% to 6% of leaf extract [38]. Deoxygenated andrographolide, Neoandrographolide, Isoandrographolide, 14-deoxy-11,12-didehydroandrographide are other underlying diterpenoids. Andrographanin, andrographolide and Neoandrographolide are isolated from roots [39].
Flavonoids: 5-hydroxy-7,8-dimethoxyflavone, 5-hydroxy-7,8,2’,5’- tetrametoxy flavone, 5-hydroxy-7,8,2’-trimethoxyflavone, 7-O-methywogonin, 2’-methyl ether were obtained as the principal flavonoids from the significant proportion of dissolved ethyl acetate of ethanol and methanol extract [40,41]. Other flavonoids extracted from root digestion are Andrographidine A, Andrographidine B, Andrographidine C, Dihydroneobaicalein, 5,2 dihydroxy- 7,8-dimethoxyflavone-2-O-β-Dglucopyranoside, 5,4΄-dihydroxy- 7,8,2΄,3΄-tetramethoxyflavone, 5-hydroxy-7,8,2΄-trimethoxyflavone, 5-hydroxy-7,8,2΄,3΄-tetrametoxyflavone, 5-hydroxy-7,8,2΄,5΄- tetrametoxyflavone, 5,2΄-dihydroxy-7,8dimethoxyflavone, 5,7,8,2΄-tetramethoxyflavone, 5,2΄,6΄-trihydroxy-7,8- methoxyflavone-2-O-β-D-glucopyranoside, wightin, 5-3΄-dihydroxy- 7,8,4΄-trimethoxyflavone, 2΄-hydroxy-5,7,8-trimethoxyflavone, 5-hydroxy-7,8,2΄,3΄,4΄-pentamethoxyflavone, 5-hydroxy-7,8,2΄,6΄- tetramethoxyflavone, 5-3΄-dihydroxy-7,8,4΄-trimethoxyflavone, 5,5΄-dihydroxy-7,8,2΄-trimethoxyflavone [42].
Phenyl propanoids and triterpenoids: In roots of A. paniculata Oleanolic acid, β-daucosterol, β-sitosterol and two phenyl propanoids such as trans cinnamic acid and 4-hydroxy-2-methoxy cinnamaldehyde were present [43].
Chemical structures of major phytoconstituents: Chemical structure of phytoconstituents shown in Figure 4 [44].
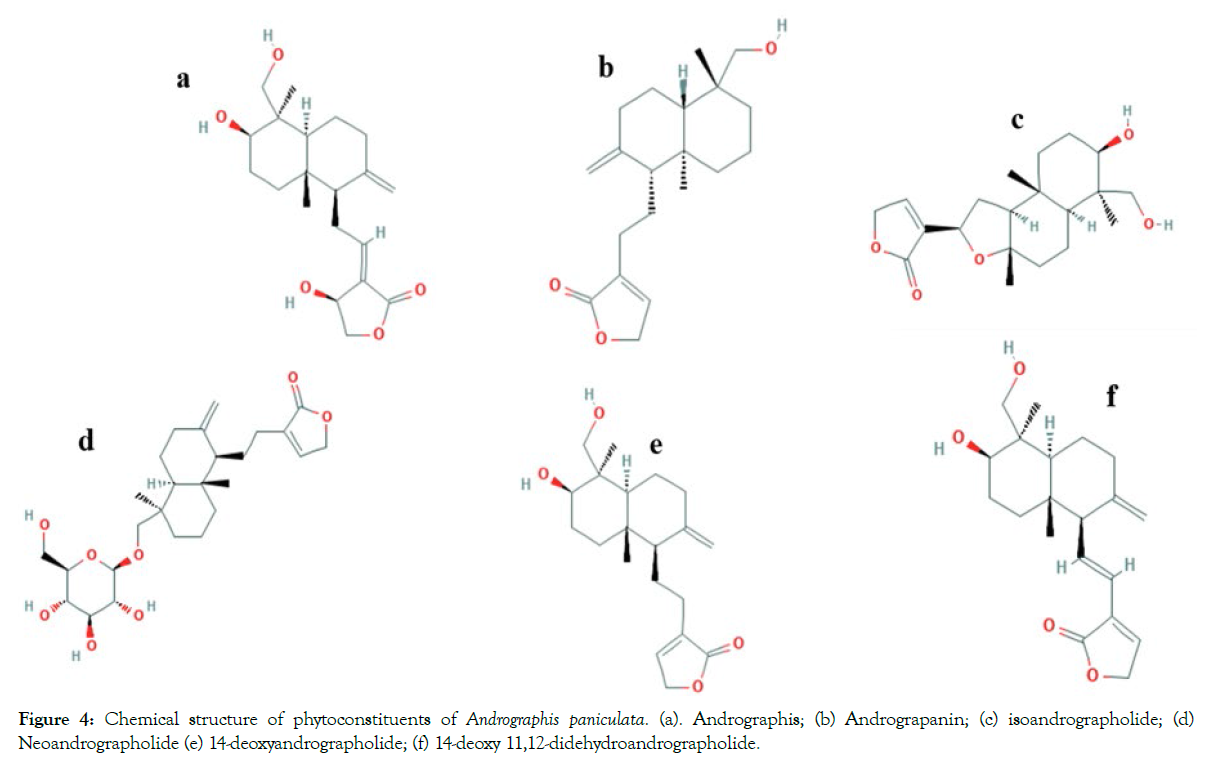
Figure 4: Chemical structure of phytoconstituents of Andrographis paniculata. (a). Andrographis; (b) Andrograpanin; (c) isoandrographolide; (d) Neoandrographolide (e) 14-deoxyandrographolide; (f) 14-deoxy 11,12-didehydroandrographolide.
Physicochemical characteristics of coarse powder of A. paniculata: The Physicochemical properties of coarse A. paniculata powder described by Sharma et al. have been summarized in Table 4.
| Color | Green |
| Taste | Intensely bitter |
| Odor | Slight characteristic |
| Melting point | 229°C |
Table 4: Physicochemical properties of Andrographis paniculata.
Pharmacological activities of constituents of A. paniculata
Antimicrobial activity: The constituents of A. paniculata were effective up against the harmful effects of the infective microbes [45]. The antimicrobic effect of A. paniculata extracts against Proteus vulgaris, E. coli, Streptococcus pneumonia and Klebsiella pneumonia was determined by performing disc diffusion method assay using ethanolic extract. The results showed that extract was efficacious against the above mention microbes by suppressing them and this restrictive action was comparable with the standard antibiotics [46].
Antioxidant activity: There are various reports supporting the evidence that flavonoids can be used as free radical scavengers and antioxidants [47]. The phytochemical analysis of extracts of A. paniculata showed significant antioxidant activity by DPPH scavenging assay. The best results were obtained with di-chloro methane (DCM) extract showing the least IC50 value of 69.32 μg/ mL. The IC50 value can be further reduced by utilizing filtered extracts. The total Lessing capacity expanded in dose dependent manner for all three extracts [48].
Anti-inflammatory activity: A. paniculata extract showed antiinflammatory activity that is credited to the ent-labdene diterpenoid andrographolide [49,50]. Many in-vivo and in-vitro research methodologies including human genomic DNA microarrays had been used to investigate the anti-inflammatory properties of A. paniculata [51].
Antidiabetic activity: The aqueous and ethanolic extract of A. paniculata shows antidiabetic activity [52]. The anti-hyperglycemic effect of A. paniculata was linked to its foremost and major contingent Andrographolide [53].
Antipyretic activity: The administration of andrographolide, neoandrographolide and deoxyandrographolide to mice, rats and rabbits through intragastric route reduce pyrexia induced by endotoxin [54]. A 300 mg of andrographolide/kg body weight showed antipyretic effects that were comparable to 300 mg of aspirin [55].
Cardiovascular activity: In rats 14-deoxy-11,12- didehydroandrographolide and 14-deoxyandrographolide had shown cardiovascular properties. Both compounds decreased the heart rate and mean arterial pressure in anesthetized rats [56,57].
Anti-HIV activity: According to several researches extricate of A. paniculata have potential effects opposite to Retrovirus and can be used in combination with modern medicines to fight against AIDS [58].
Hepatoprotective activity: A few studies related to hepatoprotective activity of A. paniculata are available. Against ethanol induce toxicity in rats multiple dose pre-treatment with arabinogalactan proteins with andrographolide was protective [59]. Hepatoprotective effects against CCl4 induced liver damage of the crude alcohol extract of leaves also reported [60].
Anticancer potential of A. paniculata: Active constituent of A. paniculata “andrographolide” is a strong therapeutic anticancer pharmacophore. It attacks the cancer cells both directly and indirectly [61].
Cytotoxic activity of andrographolide against cancer cells: Significant cytotoxic activity against culture cells of human epidermoid carcinoma of nasopharynx (KB) and lymphocytic leukemia (P388) have been shown by the methanolic extract from leaves of A. paniculata.
Cell cycle cytotoxicity induction: Inhibition of cell cycle was productively impelled on cancer cells at G0/G1 stages by A. paniculata [62]. An investigation was performed of mammalian idiopathic myeloid leukemic HL-60 cells. The investigation results showed that there was marked reduction in cell types at S and G2/M phase cells and 27 percent increase in G0/G1 phase cell types after the administration of Andrographolide (12 μg/mL) for 36 hours [63]. Cell cycle progression inhibited by andrographolide by inhibiting modulating the conformational changes of proteins (p16, p21, p27) belongs to cell-cycle and lowering the strength of cyclin A, cyclin D, CDK4, CDK2 which are required for G1 to S transition [64].
Apoptosis initiation: Induction of neuronal cell death as well as activation of extrinsic death receptor pathway (including caspase 3,8) by Andrographolide in specific individual types of cancer [65]. Recent research shows after treatment with andrographolide there was significant enhancement in apoptosis related to Tumor Necrotic Factor-α (TNF-α generating ligand (TRAIL) in numerous cancer cells of individuals [66]. TRAIL, also known as Apo2L, a type 2 membrane protein and an important anticancer agent which can induce apoptosis. It can specially assassinate cancer cell amid the usual cells and most prominent consideration for cancer associated studies and investigations [67]. Dissertations had proven that Andrographolide was additionally beneficial in combination drug management. When used along with other anticancer such as 5-Flourouracil, cisplatin, it increased the rate of apoptosis [68].
Immunostimulatory properties: A. paniculata exert strong immunomodulatory responses. The alcoholic extract and isolated andrographolide induces immune responses that are antigen specific and non-specific in nature in mice mentioned in many researches which manifest’s its potency up against many infectious diseases and carcinogens [69,70]. Andrographolide is important in controlling and maintain the productivity of components for example Interleukin-2 (IL-2), Interferon-γ (IFN-γ), Tumor Necrotic factor (TNF-α), and Natural Killer cells (NK). These factors provide protection against cancer factors. When Andrographolide was administered the antitumor venture of Immune cells towards virulence factors increases because there were significant elevations in the level of CD markers and TNF-α [71]. Andrographolide impede the cancer development by producing antineoplastic cells such as T-lymphocytes in animals [72].
Antiangiogenic and anti-inflammatory activity: As far antiangiogenic potential is concerned, Andrographolide might potentially shackle the well-defined capillary proliferations of the cancer and do not affect the normal capillaries [73].
By the administration of Andrographolide, the production of numerous onto genic components such as Endothelial vascular growth factor, nitric oxide, decreases and in addition to this there was increase in in-vivo and in-vitro IL-2 and TIMP-1 concentrations [73]. Different investigations had shown that Andrographolide holds certain restrictive actions at odds with VEGF which is chiefly and practically used by tumor cells for endurance [74]. The plant extract of A. paniculata and andrographolide both together recognized to possess anti-inflammatory properties. Impediment in the making of Reactive Oxygen Species (ROS) was a practicable procedure of the anti-inflammatory action of Andrographolide. Andrographolide turned down the levels of cyclooxygenase (CoX- 2) and Nitric-Oxide Synthase (NOS) and impede the swaddling of nuclear factor kappa B (NF- κB) to DNA [75,76].
Chemo-protective potential: A. paniculata posses’ certain properties that provide protection to the normal cells. Concanavalin A is a lectin and utilized to examine the actions of some cells such as T cells. It instigates the murine thymocytes and produces IL-2 and IFN-γ. But this production can be impeded by the administration of Andrographolide dependent on its dose and also prevented cell apoptosis produced by hydrocortisone [77]. It has been also reported that andrographolide provide protective effect against urothelial toxicity induced by cyclophosphamide [78] and oxidative injury induced by hexachlorocyclohexane [79]. In an another finding it shows that andrographolide impart armor by overpowering mitochondrial arbitrated apoptosis because the liberation of cytochrome c in the cytosol was impeded [80]. Andrographolide also provide protection to human vascular endothelial cells because it avoids adherence of intestinal tumor cells through plugging the levels of E-selectin [81].
Multitarget potential of Andrographolide: Analogues of andrographolide: several researchers have been successfully synthesize andrographolide derivatives also having anticancer potential in addition to natural occurring andrographolide [82]. By modifying the primary structure of andrographolide may improve the anti-tumor activity as described in Figure 5 [83].
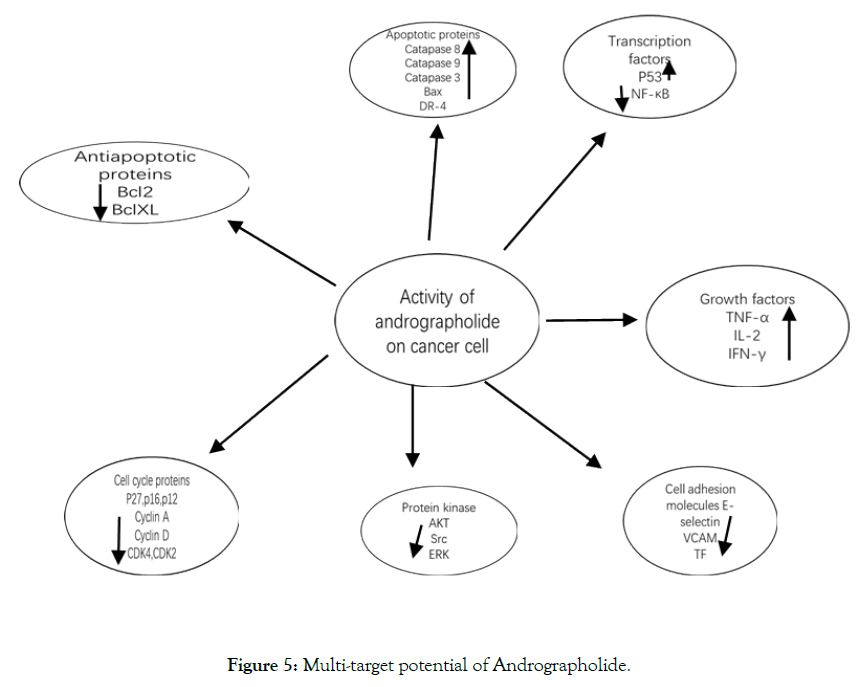
Figure 5: Multi-target potential of Andrographolide.
Pharmacokinetics of Andrographolide: Natural bioavailability of andrographolide: The Biopharmaceutical Classification system (BCS) provide information related to the solubility and permeability of drugs. It consists of four groups shown in Table 5.
| Drug class | Solubility profile | Permeability profile |
|---|---|---|
| Class I | High solubility | High permeability |
| Class II | Low solubility | High permeability |
| Class III | High solubility | Low permeability |
| Class IV | Low solubility | Low permeability |
Table 5: Different classes of drugs depending upon solubility and permeability.
Andrographolide comes under the heading of class III as described in BCS chart [84]. The investigated absolute bioavailability of andrographolide was 2.67%. The poor oral bioavailability confirms that it has high lipophilicity and its LogP value was 2.632 ± 0.135, aqueous solubility was low which was 3.29 ± 0.73 mg/mL and modification as well as outpouring of P-glycoproteins was rapid [85].
Absorption: Andrographolide with absorption half-life of 25 minutes, quickly absorbed from gastrointestinal tract into the blood. It extensively bound to blood proteins and redistributed within 1-2 hours between blood and tissues. After 1.36 hours of its administration, its maximum concentration was achieved [86].
Metabolism: The metabolites of Andrographolide are of different types such as sulfate or sulfonic acid related, creatinine related and conjugates of glucuronide [87]. In rats the significant breakdown product of Andrographolide reported was 14-deoxy-12 (R) sulfo Andrographolide [88,89]. According to these studies its metabolism is carried out by different pathways.
Excretion: Within 72 hrs of administration, almost 8.2% was eliminated through urine and the rate of elimination was 0.028 mL/min. Almost 90% of the drug eradicated by many other means one of which was metabolic transformation. During the interlude of 6 hours to 24 hours, highest rate of elimination took place [86].
Purification of active phytochemicals from Andrographis paniculata: The process may involve different approaches such as bioassay-guided fractionation, isolation assay and combinatorial chemistry. The method starts with natural extract test then suitable methods used for fractionation of active extract and tested for bioactivity by using TLC, HPLC, NMR, Mass spectroscopy [90,91]. After purification these phytochemicals are tested in-vivo and invitro to examine the anticancer effects. Detail scheme of collection, extraction, testing is shown in Figure 6.
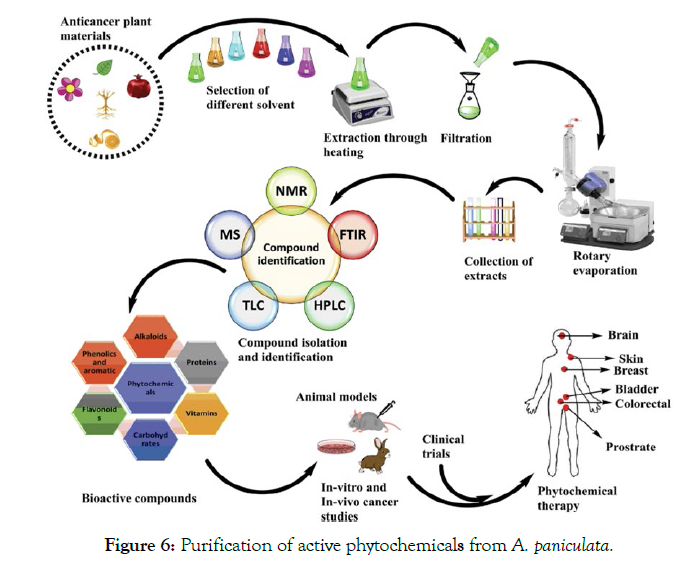
Figure 6: Purification of active phytochemicals from A. paniculata.
In area of cancer research, the search for new alternative and conventional medicines is an ongoing process. A. paniculata is renowned herbage of classical medical science, it had gained much attention due to its anti-tumor and anticancer properties. Andrographolide is a major active phytochemical of A. paniculata which possess significant anti neoplastic and immunomodulatory activities proved by numerous studies in recent times. It not only acts on various pathways to kill or eliminate the cancer but also provide protective effects to the normal cells. These characteristics of A. paniculata make it a strong candidate as a prospective plant derived anticancer drug.
The author is thankful to the handling editor and anonymous reviewers for their constructive comments and suggestion.
The authors declare that she has no conflict of interest.
Citation: Liaqat H (2021) Andrographis paniculata: A Review of its Anti-Cancer Potential. Med Aromat Plants (Los Angeles) 10: 384.
Received: 29-Apr-2021 Accepted: 11-May-2021 Published: 18-May-2021 , DOI: 10.35248/2167-0412.21.10.384
Copyright: © 2021 Liaqat H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.