Immunome Research
Open Access
ISSN: 1745-7580
ISSN: 1745-7580
Review - (2020)Volume 16, Issue 3
In 1952, the first immunoglobulin products, made from human plasma, were used to combat infectious diseases such as primary immunodeficiencies during World War II. In addition, during the last 50 years, further research has been conducted to prove whether or not immunoglobulin therapy can truly be effective against primary immunodeficiencies such as X-linked agammaglobulinemia or Common Variable Immunodeficiency Disorder through both intravenous and subcutaneous administration. Intravenous administration has been effective in increasing overall Ig serum concentration in the blood for patients with primary or secondary disorders. However, as more research was conducted, scientists had concluded that the overall cost, maintenance, and at times, lack of efficiency, makes intravenous administration a burden. Thus, scientists have looked for an alternative through subcutaneous administration. For patients with primary immunodeficiencies, subcutaneous has been proven effective in increasing immunoglobulin concentration, even more than intravenous has. The benefit of subcutaneous administration at-home, the low cost, and the heightened efficacy make subcutaneous administration far better than intravenous for primary immunodeficiency patients. However for secondary immunodeficiency patients, the efficacy of subcutaneous administration has not been fully proven and the research is scarce and unreliable. Our literary review explores the advent of immunoglobulin therapy and its past research on both intravenous and subcutaneous administration for primary and secondary immunodeficiency disorders. We sought out to find potential experimental values researchers can conduct experiments to enhance the research on subcutaneous administration for secondary immunodeficiency patients.
Antibodies; Immunoglobulin; Immunodeficiency disorders; Therapeutic interventions
In recent years, the concerns for immunodeficiency disorders are growing. For the mother ’ s infected with certain immunodeficiency disorders during gestation, they genetically pass on their ailment to the fetus: Causing the child to have a primary immunodeficiency disorder (PIDD).
Statistically, the common variable immunodeficiency disorder (CVID), affects around 1 in every 25,000 caucasianperso [1]. In these cases, patients have a severe deficiency of certain antibody isotypes, such as immunoglobulin G (IgG) and immunoglobulin A (IgA). To counteract this deficiency, a treatment known as immunoglobulin (Ig) replacement is given where prepared immunoglobulin is administered in one’ s blood either intravenously or subcutaneously. These methods vary in the way the immunoglobulin is administered, either intravenously or via muscle tissue. Ig-replacement can also be used off-label to treat other ailments, such as autoimmune disorders [2,3]. Some literature exists on antibody deficiency, but the primary focus is on PIDDs; especially since PIDDs are more common than secondary [4]. Few studies involve secondary immunodeficiency disorder, but selective research reveals Ig-replacement therapy is effective in combating infection in antibody deficiency patients. Although these studies have confirmed that intravenous Igreplacement therapy (IVIg) helps prevent infection in secondary immunodeficiency patients, subcutaneous Ig-replacement therapy may be effective in treating antibody deficiency as well. Subcutaneous is traditionally used when adverse reactions occur in IVIg therapy, and overall it can be equally effective in treating patients with secondary immunodeficiency disorder undergoing Ig-replacement therapy.
Ig-replacement therapy is used in patients with immunodeficiency disorders to replace immunoglobulin, which can prevent infection. This innovation will expand technology in medicine to be able to treat more patients and give them a better quality of life by reducing both serious and non-serious infections. The ability to introduce a new treatment variety of subcutaneous Ig-replacement will give patients with antibody deficiency treatment options and a backup option if IVIg has a poor reaction with the patient (Figure 1) [5].
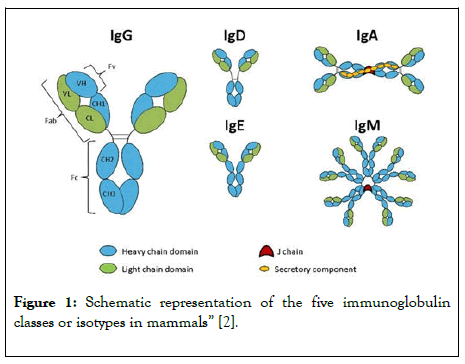
Figure 1: Schematic representation of the five immunoglobulin classes or isotypes in mammals” [2].
Past immunodeficiency research has solidified the involvement of intravenous Ig-replacement therapy as a successful treatment option for PIDDs. Research involving secondary immunodeficiency disorders is minimal, but some sources have shown how Ig-replacement therapy combats infection in secondary immunodeficiency patients as well [6]. SCIG therapy hasn’t been well researched, but some sources have suggested that it is indeed effective in reducing infections in antibody deficiency patients. Most existing research involves the phenotypical element of PIDDs, as opposed to the genotypic element and chemical compounds of both PIDD and secondary immunodeficiency disorders. This study researches the effects and success of SCIG therapy amongst patients with compromised immune systems and the resulting infection severity and number via each Ig-replacement method. Primary antibody deficiency patients often have recurrent bacterial infections, specifically of the respiratory tract. These patients are frequently born with the disorder due to its inheritance from the mother to the fetus during gestation. Patients with antibody deficiency often have recurrent infections with increased morbidity and mortality [7]. Secondary immunodeficiency disorder develops in patients because of numerous possible factors: hematological malignancies, specifically lymphoma, immunosuppressive medication, chemotherapy for B lymphocytes, infection, and corticosteroid treatment [8]. This disorder can be a result of the malignancy or a method of treatment for the malignancy. There is a greater diagnosis delay in secondary immunodeficiency patients compared to PIDD patients. These patients have inadequate production of antigen isotypes, which are different types of antibodies. Antibodies are made up of immunoglobulin molecules. Each antigen isotype is responsible for different mechanisms in the body [9]. All of these isotypes are vital to the immune system since they are produced by plasma cells to phagocytize antigens in the body, without treatment these patients are vulnerable and susceptible to infection [10]. In the human body, there are 5 immunoglobulins: IgM, IgG, IgA, IgD, and IgE. The subclasses of IgG are Ig1-4 and there are two subclasses of IgA are IgA1 and IgA2. The main cause of antibody deficiency is stemmed from the lack of specific enzymes in B cell development which eventually leads to impaired antibody response and fails to produce immunoglobulin. It can also be caused by T cell lymphocytes failing to activate the aforementioned B cells. In diseases such as X-linked agammaglobulinemia, the failure of B cell maturity is caused by mutations of the protein tyrosine kinase, and the same failure of maturity, or malfunctioning of gene switching, causes a lack of immunoglobulin. In most primary immunodeficiency disorders, there is a severe lack of immunoglobulin G. In primary deficiency disorders such as specific antibody deficiency, there are abnormal IgG antibody responses. Or, in patients with IgG subclass deficiency, there are normal total levels of IgG, however, the subclasses are at a deficiency. It is not the only IgG that is at a deficiency, but also IgA (as seen in selective IgA deficiency where their levels are below 0.07 g/L). Whenever patients are identified with such low levels, they are given immunoglobulin therapy either intravenously or subcutaneously [11,12].
Ig-replacement therapy is a blood infusion of immunoglobulin antibodies that will give the body the necessary antibodies to fight infections. This treatment is used in immunocompromised patients that don't have the antibodies to phagocytize antigens. This therapy gives the patient the immunoglobulin they need to protect themselves, in theory. Ig-replacement therapy is often used intravenously on PIDD patients, but only select studies have found secondary immunodeficiency disorder patients to have decreased severity and number of infections after IVIg replacement therapy. In almost all patients, mortality decreased following IVIg therapy [13]. IVIg is when the immunoglobulin is infused via the vein. SCIG therapy is when it is infused right underneath the skin. In both primary and secondary patients, Ig-replacement therapy has been found successful in decreasing the number and severity of infections in antibody deficiency patients [14]. The doses, for patients with Common Variable Immunodeficiency Disorders, have around400-600 mg/kg per one’s body weight (every weeks) [15]. The IgG concentration should be above 5 g/L although some people benefit from 7 g/L, and the normal IgA concentration should be around 0.7 g/L to 4 g/L. In 1952, the first report of intravenous immunoglobulin therapy against agammaglobulinemia, a primary infection, was conducted by Dr. Ogden Bruton. In this case, the patient’s antibody response was impaired, as well as a complete absence of gamma globulin. However, a subcutaneous serum was found to have improved the patient’s conditions, and in recent years, both intravenous and subcutaneous administration of immunoglobulin has been found to improve the dangers of primary immunodeficiency disorders Intravenously, prior experimentation has been done for both primary and secondary disorders. In a study analyzed by Dr. Elena Perez children with hypogammaglobulinemia were “treated with 400 mg/kg every 3 weeks for 2-3 months and followed up for 1-3 years” [16]. The conclusions were that the frequency of overall infection decreased from 0.39 to 0.0047 infection rate per month per child. In addition, there was a 91% to 21% decrease in overall hypogammaglobulinemia infection for children administered with IVIG. In separate studies, intravenous immunoglobulin was also given for selective IgG subclass deficiencies, as they had an impaired antibody response. Three studies were conducted: a 10 patient open-label study, a 17 patient retrospective study, as well as a 132 patient retrospective study. 16 All of these patients had subclass deficiencies. In the open-label study, the quality of life for 10 patients with infection, and the need for antibiotics were improved throughout the 12-month treatment with IVIG rather than 3 months without IVIG. In the retrospective study with 17 patients, 4 were administered prophylactic antibiotics while the other 13 were treated with IVIG. Within these 13, 2 patients chose not to respond to their quality of life after treatment, 6 had reported having “ dramatic ” improvement from constant infections, while 5 had reported having “ moderate ” improvement. This shows how 84% of patients were said to have improved, therefore further concluding that IVIG is an effective method. In the 132 patient retrospective study, 92 patients were reported to have an >50% “reduction in the rate of respiratory tract infections requiring antibiotics (p<0.001), and the overall reduction rate in respiratory tract infections was 61% (p<0.001) ”. Although the usage of IVIG is scarcefor IgA deficiencies, IgG2 subclass deficiencies or impaired IgG production, often exist with IgA deficiencies making it nearly imperative to receive IVIG treatment. However, it is worth noting that for some patients who are IgA deficient, who produce IgE anti-IgA antibodies, anaphylaxis may occur when immunoglobulin is administered intravenously. When adverse reactions occur, such as the aforementioned anaphylaxis, subcutaneous may be effective. In experimentation conducted by Dr. Gustafson and his team, SCIG (subcutaneous immunoglobulin therapy) was seen to have several benefits including an overall improvement in lifestyle, as well as an increase in IgG concentrations. The method they used was to observe 12 patients with several primary disorders such as CVID, or XLA, who were already under at-home subcutaneous treatment of 100 mg/kg body weight. These infusions were done 12 times bi-weekly, therefore, 144 infusions were done in total at-home. They had concluded that the levels of IgG were mostly high throughout the therapy [17]. In a separate study conducted by Dr. IsilBarlan and his team observed 16 patients (9 male and 7 female) who were initially given IVIG. These patients were given IVIG in dosages of 0.33-1.25 g/kg every 2-4 weeks, and then afterward, were given SCIG 0.03-0.43 g/kg every one-two weeks with 10% immunoglobulin concentration [18].
Results were as followed with IVIG
The IgG levels were at a median of 241 mg/dL.
The lowest value was 90 mg/dL.
The highest value was 598 mg/dL.
Results were as followed with SCIG
The IgG levels were at a median of647 mg/dL.
The lowest value was 230 mg/dL.
The highest value was 1028 mg/dL.
The p-value is less than 0.043 (statistically significant comparedto0.05 with IVIG).
These values have shown that SCIG is more effective in increasing IgG concentrations over acertain period. In addition, the overall infection rate was decreased as well and there were minimal side effects involved. However, although the results conclude that SCIG is more effective in increasing IgG concentrations, these results are only for patients with primary immunodeficiency disorders.
When it comes down to secondary, the research is vague, and not much experimentation has been done: especially for secondary subcutaneous. In one particular study conducted by Health Quality Ontario, SCIG treatment was administered weekly at home. In the studies they analyzed, many of the outcomes were positive. When it came down to the infection rate, the SCIG rate was between 0.03 and 0.19 (lower than the FDA target of below 1). In addition, overall antibiotic usage was minimal for SCIG patients as well. In a study conducted by Compagno et al., they reported that antibiotic usage for SCIG was every 1.43 cycles per year, and IVIG was 1.82 cycles per year [5]. In a separate study by Fasth and Nystrom, they “reported 3.5 and 12.8 days of antibiotic use for SCIG and IVIG, respectively”. The reports have also stated that “home-based SC infusion is safe and effective, with clinical outcomes that are comparable to the clinical outcomes of hospital IV infusion” [19]. In addition to this, throughcost-minimization analysis, the overall cost of at-home SCIG is much cheaper and can improve daily life for patients as they do not need to visit hospitals frequently. However, the report conducted also states that the quality of evidence is low, meaning that further experimentation will be more beneficial. There is controversy amongst medical professionals and researchers about the effectiveness and safety of this procedure. There are two extremes of data in diagnostic delay and age of onset. There is also a lot of information that still needs to be explored involving causation of immunodeficiency disorders, involving the study of chemical composition and genetics. More studies could be completed involving a mass, international cohort that would give researchers a better idea of the phenotypic aspects of the disorder and a better idea of how to define and treat the disorders [20].
In a perfect setting, with viable resources and time, we would experiment with subcutaneous administration impacting patients with secondary immunodeficiency disorders. We would model our experimentation off of Dr. Hoffman and his team’s research on home-based subcutaneous administration for primary immunodeficiencies [21]. Their methods involved treating 82 patients with a weekly SCIG dose of 100-200 mg/kg per one ’ s body weight at a concentration of 16% immunoglobulin. This treatment was conducted over 9 months which, in a perfect setting, we would model. Amongst these 82 patients, 54% of them had CVID, 16% had X-linked agammaglobulinemia, and only 9 patients had secondary disorders which, as the researchers implicitly stated, is not a large enough sample to prove subcutaneous administration had significant effects on SIDD’s. With the aforementioned dosage amounts, as well as Ig concentration, we can replicate this experiment but increase the sample size of patients with secondary immunodeficiencies. As stated in the literary analysis, the report conducted by Health Quality Ontario explicitly stated that the quality of their evidence was low, and there were certain questions regarding the safety and efficacy of this procedure. With regards to Dr. Hoffman’s team, “the SCIG therapy was assessed as "excellent" in 89% of patients. 10% of patients, the SCIG therapy was assessed as "acceptable", and in only one patient was the SCIG therapy assessed as insufficient”. With this evidence, in a perfect setting, we can conduct two possible experiments:SCIG administration of 0.03-0.43 g/kg (30-43 mg/kg) every one-two weeks with 10% immunoglobulin concentration for secondary immunodeficiencies.
In the experiment conducted by Dr. Barlan stated in the literary analysis, these values have led to favorable results with an overall increase in IgG concentration for patients with primary immunodeficiencies.With solid evidence from Dr. Hoffman’s team claiming SCIG was, at a majority, excellent for PIDD’s, as well as evidence from Health Quality Ontario suggesting that a home-based infusion of SCIG for secondary may give favorable results, we can conduct an experiment focusing on 12 patients (same number used in Gustafson’s experiment) with secondary immunodeficiencies such as chronic lymphocytic leukemia or non-Hodgkin lymphomas, and see whether or not the aforementioned dosage amounts and concentrations would be effective [17,19,21]. However, one note to consider according to Dr. Perez and her research on SCIG primary, is that “doses may need to be further adjusted in patients with a very low or very high body mass index”. This information can be crucial when conducting on patients with SIDD’s.
2. SCIG administration of 100-200 mg/kgper one’s body weight weekly at a concentration of 16% immunoglobulin for secondary immunodeficiencies.
In the experiment conducted by Dr. Hoffman and his team, these dosage amounts were proven to be successful for patients with primary immunodeficiency disorders (in order to see if subcutaneous was a viable alternative) [21]. However, this experiment was also done with a small sample size of patients with secondary disorders. Increasing the sample size, from 9 patients to potentially all 82 (in Gustafson’s experiment, rather, 70% of all 82 had PIDD’ s ), could be beneficial in seeing whether or not subcutaneous has a clear effect [22].
In a separate experiment conducted by Dr. Maria Dimou and her team, they used a c facilitated subcutaneous administration for secondary deficiencies at 10% IgG concentration at a dosage of 0.4-0.8 g/Kg/month [23]. They conducted over 962 infusions. The results yielded positive results as “thirty (39) pts (86.7%) had no adverse drug reaction ADR, except mild edema at the site of injection for 8-24 hours after the infusion ” .However, the evidence is scarce as specific immunoglobulin concentrations were not reported, and the safety and efficacy were also not reported. With such a large sample size, it is difficult to capture all results. Lowering the sample size to a number smaller, as we provided in our two possible experiments, could potentially give solid IgG concentration numbers and better data. Nevertheless, Dr. Dimou’s experiment provides more support, and confidence, to conduct further research [23].
In all the prior experiments, in a perfect setting, we will base off our administration through Dr. Dimou ’ s variables and equipment: a variable rate portable pump and a subcutaneous 24G needle. In a professional testimony given by Jihad Younes, a doctor in allergy and immunology who owns Allergy and Asthma Treatment Center, a private practice in Lake Orion, Michigan with Troy Beaumont as an affiliated hospital, Dr. Younes stated that “ideally you want to transfuse every 3 weeks, now they are doing it at home with subcutaneous, every week, so that’s even better. That means you keep a steady level. If you let it go down, you will have a problem. It’s not going to work as well, so if you do the calculation, it's going to take about 6-9 months to really drop your immunoglobulin levels to the point where your levels so you know you are going to have a problem”. In order to further our experimentation and collect strong data, in a perfect setting, the experiment would take 9 months, with at-home administration and weekly or biweekly administration.
To further establish that subcutaneous administration could potentially be useful, we interviewed Dr. MNV Ravi Kumar. He has a Ph.D. in Chemistry and is a professor of pharmaceutics at Texas AandM who researches management, prevention, and treatment of disease. In his professional testimony, he stated that “subcutaneous would be more beneficial than intravenous because of how it's administered”. Because subcutaneous can be administered at home without the guidance of medical practice rather, it can be easier for patients and since it is readily available and can be taken weekly, total IgG concentration can potentially increase for primary disorders. Regarding secondary, he had also stated that research was scarce, and evidence was not strong. He stated: “ secondary is less researched because of marketing, rarity, lack of test subjects, small demand, and lack of interest by large companies”. He also added that the cost of this therapy was quite high, even though subcutaneous is relatively cheaper than intravenous. Through these experiments, in a perfect setting, we can hopefully further the advent of immunoglobulin therapy by looking at different alternatives such as subcutaneous administration for secondary disorders. In addition, we can also try to find alternatives to mitigate costs and truly see an increase in a potential cost-benefit analysis.
Professional testimony given by Jihad Younes, a doctor in allergy and immunology who owns Allergy and Asthma Treatment Center, a private practice in Lake Orion, Michigan with Troy Beaumont as an affiliated hospital Immunoglobulin therapy was first discovered as an effective treatment when a boy, who was having a lot of infections, had the test, protein electrophoresis. Protein electrophoresis “is basically a study on the proteins'', and this boy’s doctor found that some of his proteins were missing, the gammaglobulins. Gammaglobins are the different antibody isotypes, with IgG as the main antibody. Since this boy had fewer IgG antibodies, they gave him this treatment to see what happened. This treatment was found to be successful, and he survived. “ Doctors called this lack of IgG antibodies, agammaglobulinemia.
And these disorders you can treat with immunoglobulin replacement.” No treatment is guaranteed, so “it’s certain that some people will die from agammaglobulinemia”. The immune system works differently for everyone, so there’s no way to know if the treatment will be successful. Another patient was diagnosed with immunodeficiency disorder only because he had other disorders such as arthritis and joint swelling. “Part of the rheumatology workup, they ordered immunoglobulin levels, and they were very low” in this patient, so this is how they diagnosed him. The kid wasn’t experiencing any symptoms, so this revealed in some patients they were asymptomatic, but still had low antibody levels. “Many people are over-treated; you find that they have low levels with no infection, and someone starts them on immunoglobulin treatments when it wasn’ t justified. In those cases, you won’t get much improvement because, to begin with, you didn’t have much of a problem”.
He also added that “most people don’t show common variable immunodeficiency until they’ re in their 40s, 50s, or 60s. It could take 10-12 years to be diagnosed since the levels begin to drop gradually”. This problem with early, or late-onset can cause a potential problem in the diagnosis itself and the therapy might not be of use at a certain point. With diseases rather than CVID, such as agammaglobulinemia, “kids can be normal at birth, for the first 6-9 months of life they are fine. They are fine because they have maternal antibodies, so as long as you're born full term, if you're born premature, 28 weeks of gestation, you don’ t have maternal antibodies. The babies are considered immune deficient”. Dr. Younes finally stated that “ in immunodeficiency patients, it’s not clear; they don’t have one problem, they have a lot of autoimmune issues, so they can have arthritis, inflammation of the thyroid, anemia, low platelet counts, all because of autoimmune problems. There is a relationship between immune deficiency and autoimmune disorders. It's called immune dysfunction, your immune system isn’ t working properly. ” With this following information, scientists can find the proper prognosis and treat patients from all sorts of ages (ranging from children to adults) (Figure 2).
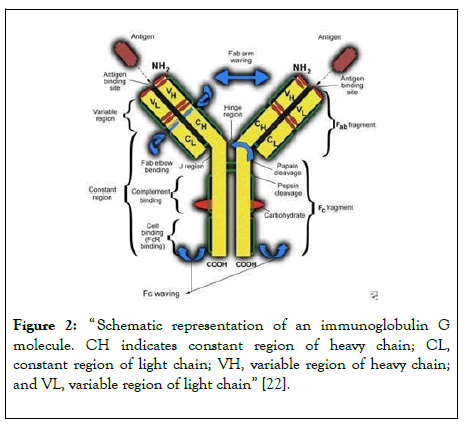
Figure 2: “ Schematic representation of an immunoglobulin G molecule. CH indicates constant region of heavy chain; CL, constant region of light chain; VH, variable region of heavy chain; and VL, variable region of light chain” [22].
Professional testimony given by Dr. MNV Ravi Kumar. He has a PhD in Chemistry and is the professor of pharmaceutics at Texas AandM who researches management, prevention, and treatment of disease. In a separate interview with Dr. MNV Ravi Kumar, he went in depth to talk about how subcutaneous administration works and why it’s so useful. Right from the start, he stated how “subcutaneous would be more beneficial than intravenous because of how it's administered”. This is due to the sheer fact that subcutaneous is “self administrable”. In addition, subcutaneous can be even more effective due to how. “ there will be a higher concentration of immunoglobulin because [subcutaneous administration is under the fat ”. This is due to the fact that “subcutaneous fat acts as a barrier”. With subcutaneous, “ diffusion is faster, so less doses are more effective”. With this information, we decided to put forth the two potential experiments that can be conducted with low dosage amounts compared to other dosage amounts in prior research documentation. In addition, with regards to overall cost, he states how because subcutaneous is self administrable, “the cost is going to go down because you don't have to have doctors or nurses to administer this treatment”.
He states: “the moment you remove expertise as a requirement for administration, there will be a significant cost reduction, and the bioavailability of this compound is going to increase, and dosage can be cut down ” . However, he does mention that secondary immunodeficiencies (with subcutaneous treatment) are “less researched because of marketing, rarity, lack of test subjects, small demand, and lack of interest by large companies”.
In Dr. Barlan and his team ’ s research on subcutaneous administration for primary disorders, the results were positive. As previously stated:
- The IgG levels were at a median of 647 mg/dL.18
- The lowest value was 230 mg/dL.18
- The highest value was 1028 mg/dL.18
- The p-value is less than 0.043 (statistically significant compared to 0.05 with IVIG).
In addition, on a smaller scale for one patient, during IVIG this specific patient had a serum IgG level of 280 mg/dl at a dosage of 0.8 g/kg every two weeks. When IgG was administered subcutaneously, IgG serum levels increased to 1040 mg/dl in the 8th month of treatment for the patient. According to Dr. MNV Ravi Kumar, the main reason due to an increase in Ig concentration for subcutaneous is largely due to the immunoglobulin being administered in subcutaneous fat itself (because subcutaneous fat acts as a barrier and can help facilitate diffusion easier).
This steady increase of IgG through subcutaneous methods for a patient with a primary disorder is what we would be trying to achieve for secondary patients in both plausible clinical trials.
Due to the complexity and rarity of immunodeficiency disorders, there is minimal research regarding primary and secondary immunodeficiency disorders. This research is conducted based on past case studies, statistical analysis, professional testimony, and observation of trends. Immunoglobulin replacement therapy is expensive, so many patients aren’t able or aren’t willing to risk trying Ig therapy as a treatment option due to the uncertainty of its effectiveness. This limits the opportunity for all patients, although few since the rarity, to get this type of treatment. Ig therapy and antibody deficiency are hard topics to find and conduct research on. In the future, researchers may be able to find a way to decrease costs associated with this kind of treatment or discover new, effective treatment options for patients. Dr. Younes gave insight to alternative treatment options such as a bone marrow transplant, but it is highly risky and hard to come by. Because of the rarity, the limited research that is conducted is vital for these patients with these disorders. In the context of the expensive treatment, limited case studies, and rarity of the disorder, more research should be done since the studies conducted aren't a great representation for all patients. More research should be conducted involving secondary immunodeficiency disorders specifically so new findings can be confidently made in the future. Secondary immunodeficiency disorder has been researched less compared to primary immunodeficiency disorder, so more researchers should strive to look into these topics more closely to be able to find a more effective, affordable treatment option.
“Ideally you want to transfuse every 3 weeks, now they are doing it at home with subcutaneous, every week, so that’s even better. That means you keep a steady level. If you let it go down, you will have a problem. It’s not going to work as well, so if you do the calculation, it's going to take about 6-9 months to really drop your immunoglobulin levels to the point where your levels so you know you are going to have a problem.” There are so many possibilities for research within the realm of antibody deficiencies and immunoglobulin replacement. It is possible to have intravenous and subcutaneous treatment methods for treating both primary and secondary immunodeficiency disorders.
We acknowledge the support of Dr. Ravi Kumar and Dr. Younes for their personal testimonials. In addition, we would like to thank Aatmi Mehta, our mentor, who we sought out for advice and information regarding certain elements of our research.
Paige Gordon is a rising senior at Rochester High School in Rochester, Michigan. She’s interested in medical research and pursuing a career in the medical field. Gordon aspires to give people answers in the form of a medical diagnosis as a future physician. She plans on continuing her studies in college by majoring in biomedical sciences with a minor in Spanish, and eventually attending medical school. Her passion for immunology, in particular, has driven her to research this topic.
PranavVadlamudi is a rising junior at Shrewsbury High School in Shrewsbury, Massachusetts. He has a deep passion for the field of medicine and he plans on pursuing a career in the medical field. Vadlamudi seeks to provide people with the proper care and need, as well as effectively communicating with patients as a future physician. He hopes to potentially major in biological sciences, or psychology.
Citation: Vadlamudi P, Gordon P (2020) Efficacy of Subcutaneous Immunoglobulin Replacement Therapy in Treating Secondary Immunodeficiency Disorders. Immunome Res. 16:6074. DOI: 10.35248/1745-7580.20.16.6074
Received: 14-Aug-2020 Accepted: 28-Aug-2020 Published: 04-Sep-2020 , DOI: 10.35248/1745-7580.20.16.6074
Copyright: © 2020 Vadlamudi P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.