Entomology, Ornithology & Herpetology: Current Research
Open Access
ISSN: 2161-0983
ISSN: 2161-0983
Research - (2020)Volume 9, Issue 3
There is need for a simple, cheap and efficient collection and control tool for the different stable fly-species in hyperinfested foci. The efficacy of a modified unbaited Vavoua trap (MVT) was compared with an unbaited Nzi as a standard. Each trap type was set either inside or outside the cattle overnight pen with rotation taking place after four days for eight days in September 2018 in Ngaoundere. Fly collection was made every 24 hours and sorted using standard identification keys. In total, 2105 hematophagous flies were caught and constituted of 1417 S. niger niger (89 S.n/t/d), 124 S. calcitrans (8 S.c/t/d), 562 Musca spp. (25 m/t/d) and 2 Chrysops distinctipennis (0.1 Cd/t/d). The overall mean catches with the MVT (50.96 ± 57.16) was higher than with the Nzi (27.43 ± 27.73), with no statistically significant difference (t=0.9, df=46. 0, P=0.37). Higher mean catches were recorded outside the overnight cattle pen (47.79 ± 55.27) than inside (39.83 ± 55.40) with no statistically significant difference (t=0.5, df=46.0, P=0.62). Stable flies and tabanids were highly caught outside the overnight stable, while Musca spp. were highly caught inside. In conclusion, the MVT had similar efficacy and specificity as Nzi and can serve as a simple and efficient tool for the survey and control of stable flies in the Adamawa plateau and elsewhere.
Modified vavoua; Nzi; Efficacy; Stable flies; Tabanids; Ngaoundere
The control of most haematophagous insect vectors especially Stomoxyinae can be effectively conducted using collective approaches such as sanitation, biological, mechanical and chemical [1]. Talking of biological control, Baleba [2] reported on the potential pathogen, predators and parasites that can control stable flies. The chemical approach includes the use of insecticides. Due to the high biting insect infestation rates of livestock farms in Ngaoundere, farmers have devised certain control measures like bush fires and use of insecticides (veterinary and agricultural)to manage flies [3,4].
The use of insecticide incorporated screens is a major tool used by the Special Mission for Tsetse Erradication (MSEG) to control tsetse and other biting flies in Ngaoundere and other infested areas and its efficacy to control tsetse and other dipterous insect vectors in the Adamawa and North regions of Cameroon has already been reported [4,5]. Some insecticides have been reported to be resistant to stable flies [6,7], therefore it is important to consider an alternative approach. The physical or mechanical control approach is based on the use of traps like box trap supplemented with alsynite fibre glass sticky panels [8] and was reported to be efficient for the control of stable flies. The blue-black cloth traps particularly the Vavoua trap has been reported to be highly specific tostable flies [9-11]. According to Solo´rzano [12] the Nzi is more efficient than Vavoua in the collection of stable flies, but the Nzi is large and need several tension points that makes it very difficult to transport and set up in the field.
Also, the Vavoua trap is very efficient, expensive and not commercially available in local markets. Therefore, there is need for an efficient, simplified and available trap to farmers for the control of stable flies and subsequently reduce the diseases they transmit. This study focuses on the design of a simple blue black trap known as the modified vavoua trap (MVT) as well as to compare its efficacy and specificity with the Nzi trap as the standard.
Study site
Ngaoundere is the head quarter of the Adamawa region. It falls within the following geographical limits, latitude 6° and 8° North of the equator and longitude 11° and 15° East. Ngaoundere falls in the tsetse free belt of Cameroon. It has a Soudano-Guinean climate with two seasons (rainy and dry). The vegetation of Ngaoundere ranges from savanna, gallery forest and secondary forest. Ngaoundere is host for the main cattle market of the region and is in Mbidjoro that constituted our study site.
Mbidjoro is 15 km from the town of Ngaoundere along the Tibati motorable high way. The study site constituted of a mixed cattle herd with three breeds (Goudali, Akou, Charolais and Holstein) and their cross breeds (metis). The cattle overnight pen was located 50 m to 100 m away from four poultry farm buildings. The poultry blocks were not fly proof. Eight herds of cattle were found in the sampling area. The cattle density of the study site was 241 cattle heads/7 Km2.
Design of the Vavoua trap
The different textile parts of the trap must be cut so as to use the maximum amount of fabric from the piece. Black screens (76 × 28 cm): in a 100 m by 1.80 m wide piece (Figure 1). The blue screens (trapezium like, 76 × 48 × 27 cm) cut head to tail (Figure 1B): in a 100 m piece by 1.50 m wide; the 15 cm drop, after longitudinal stitching, still provides a considerable number of screens (Figure 1G). The cone is mounted in three parts, each with an apex angle of 56° and a radius of 78 cm and slant height of 85 cm (Figure 1F).
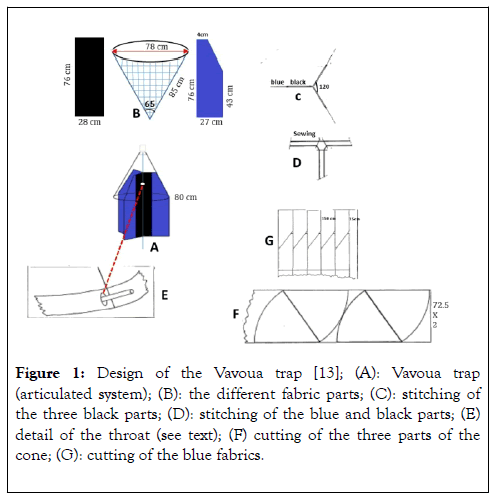
Figure 1. Design of the Vavoua trap [13]; (A): Vavoua trap (articulated system); (B): the different fabric parts; (C): stitching of the three black parts; (D): stitching of the blue and black parts; (E) detail of the throat (see text); (F) cutting of the three parts of the cone; (G): cutting of the blue fabrics.
Assembly of the three black screens: two of the screens are superimposed and then sewn longitudinally 13 cm from the edge; an identical seam is made between the 1st screen and the 3rd arranged along the opposite edge; finally the 2nd and 3rd screens are sewn together in the same way (Figure 1D). This creates a 2 cm groove for the passage of the fixing material and the trap (Figure 1D). Between the free edges and two black screens, insert a 0.5 cm blue screen, aligning the upper edges, then sew longitudinally (Figure 1C).
The internal part of the trap thus produced is then sewn with the three sectors of the cone: the screens are then placed at 120°. For capture, the end of the cone is truncated to allow the lacing support system to pass. For the cone, it is protected from the metallic axis by a carded cotton pad; a piece of string around the top holds the whole. The rigidity of the cone can be ensured by a circular galvanized wire of 80-81 cm in diameter (Figure 1A). When setting up, the bottom of the trap must be between 30 cm and 40 cm from the ground. The length of the iron rod (8 mm concrete iron) will essentially depend on the nature of the site: it is advisable to use 1.50 m iron rod as support.
Design of the Modified Vavoua Trap (MVT)
The MVT [14] is a simple blue/black polyester trap with a trapezium-like blue/black core in the mosquito net section. The section of the trap protruding to the bottom of the mosquito net is in the form of a blue-black flat screen. The size of the MVT is 132 cm × 84 cm (Figures 2A-2E).
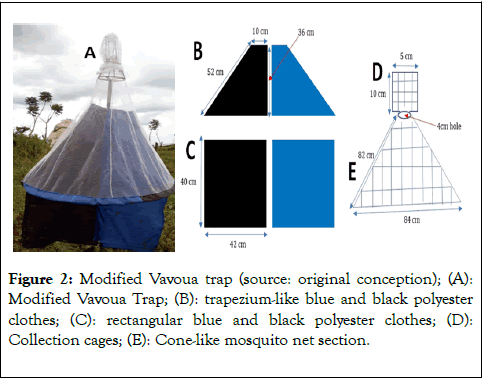
Figure 2. Modified Vavoua trap (source: original conception); (A): Modified Vavoua Trap; (B): trapezium-like blue and black polyester clothes; (C): rectangular blue and black polyester clothes; (D): Collection cages; (E): Cone-like mosquito net section.
This trap has the same trapping mechanism as the Vavoua and Nzi. Its ability to capture dipterous insects relies only on the visual cue offered by the blue and black colors of its cloth. Concerning the specific dimensions of this trap, the trapeziumlike blue-black pieces each has the following dimensions: base length of 42 cm, length of the top is 10 cm, vertical height is 36 cm, and the slanting height is 52 cm (Figure 2B).
The rectangular blue and black pieces each have a dimension of 40 cm height and 42 cm width (Figure 2C). The mosquito net sewn to cover the collection cage has a dimension of 10 cm height and 5 cm width (Figure 2D). The mosquito net section designed in the form of a cone has a bottom diameter of 84 cm, a top diameter of 4 cm, and a height of 82 cm (Figure 2E). The entire trap is held by a vertical iron rod of 1.5 m in length. The distance from the ground to the trap must be maintained at 20 cm (Figure 2A).
The Nzi trap
The Nzi is a blue-black trap like the MVT but is larger in size (Figure 3). This trap is originally designed by Mihok at ICIPE. This trap consists of a 1 m × 1 m mosquito net. Blue and black cloth pieces of 0.5 m × 1 m in dimension that are sewn to form the wings of this trap (Figure 3). The horizontal net shelf forms the roof of this trap and holds the collecting cage. The trap is supported by four sticks or iron rods with at least six tension points that makes the trap very cumbersome.
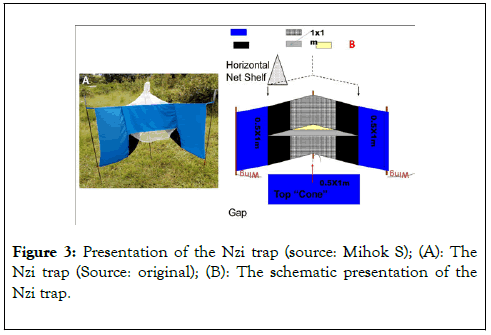
Figure 3. Presentation of the Nzi trap (source: Mihok S); (A): The Nzi trap (Source: original); (B): The schematic presentation of the Nzi trap.
Study design
Unbaited Nzi and Modified vavoua (MVT) traps were pitched; one inside the cattle overnight stable and the other outside the grazing field (Figure 4). The two trap types were 500 m from each other. A 2 × 2 Latin square with rotation experimental design was used.

Figure 4. The position of traps; (A): inside the cattle pen; (B): outside the cattle pen.
The cages of the traps were emptied every 24 hours. Flies were collected into well labeled plastic bags for identification using a dissecting microscope. The trapping effort was 2 traps × 8 days in the rainy season (August to September)=16 traps days.
Fly identification
Stomoxys spp. were identified using the key of Zumpt [15]. Tabanids identification was realized using the morphological key of Oldroyd [16]. The identification of the genus Musca was made using the key of Gregor.
Data analysis
The abundance was defined by the Trap Apparent Density (ADT) known as the number of Stomoxys caught per trap and day as follows:
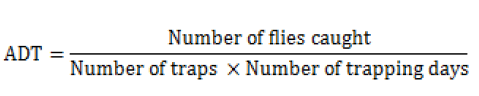
The specificity was calculated using the following formula:
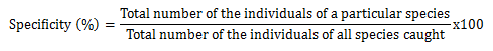
The JASP statistical software of version 0.8.5.1 was used for the statistical analyses. The mean catches of the trap types and their positions were compared using the Student t-test. The statistical test was kept at the p<0.05 significant level.
The entomological prospection resulted in 2105 flies with the following flies identified with their apparent abundances in order of magnitude: 1417 S. n. niger (89 S.n/t/d), 562 Musca spp. (25 m/t/d), 124 S. calcitrans (8 S.c/t/d) and 2 C. distinctipennis (0.1 Cd/t/d).
Catches with respect to trap type
The MVT caught muscids (Musca spp. and Stomoxys spp.) but failed to catch tabanids. The Nzi trap caught all flies including Musca spp., Stomoxys spp., and Chrysops (Figure 5). The number of muscids caught by the MVT was higher for each species than with the Nzi.
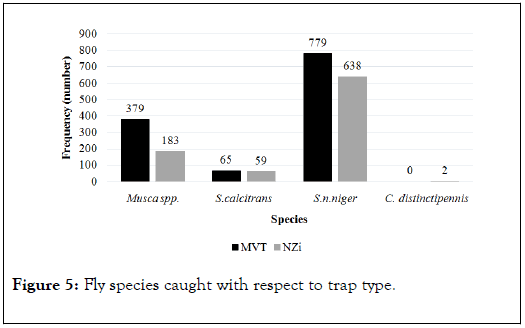
Figure 5. Fly species caught with respect to trap type.
The specificity of the MVT was higher for S. niger niger (54.97%) and S. calcitrans (52.42%) as compared to Nzi, but the Nzi trap recorded 100% specificity for C. distinctipennis and zero with the MVT (Figure 6).
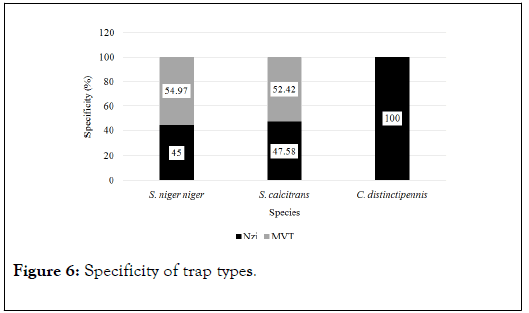
Figure 6. Specificity of trap types.
Fly catches with position of traps
The Musca spp. count was higher outside the cattle pen than inside (Figure 7). Stomoxys calcitrans had the same catches when traps were pitched either inside or outside the cattle pen. Stomoxys niger niger catches were higher outside than inside the cattle pen.
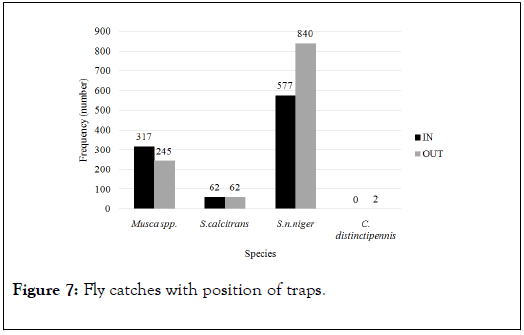
Figure 7. Fly catches with position of traps.
Efficiency of trap-types based on mean catches
The mean catches with trap types revealed a higher mean catch with the MVT (50.96 ± 57.16) as compared to the Nzi (27.43 ± 27.79) even though there was no statistically significant difference (t=0.9, df=46.0, P=0.37) with catches of the two trap types.
Regarding the position of the trap types, there was a higher mean catch outside (47.79 ± 55.27) the cattle pen than inside (39.83 ± 55.40) with no statistically significant difference (t=0.5, df=46.0, p=0.62) (Table 1).
| Parameters | Number | Mean ± Standard deviation |
|---|---|---|
| Trap models | t=0.9, df=46.0, p=0.37 | |
| Nzi | 882 | 27.43 ± 27.79 |
| MVT | 1223 | 50.96 ± 57.16 |
| Position of trap models | t=0.5, df=46.0, p=0.62 | |
| Inside | 956 | 39.83 ± 55.40 |
| Outside | 1149 | 47.79 ± 55.27 |
Table 1: Mean fly catches based on trap models and position.
The occurrence of tabanids and Stomoxyini in the rangeland of Ngaoundere has already been reported [17]. In our study, high numbers of stable flies were caught outside the cattle pen as compared to the inside. The high catches outside could be due to the proximity of the traps to the breeding sites of the stable flies [18,19] because stomoxyines developmental substrates consist of faecally-soiled surfaces and decomposing vegetation as well as beddings from poultry [2] and such potential breeding material were found outside the cattle overnight pen where cattle graze as compared to the muddy nature of the inside of the cattle overnight stable. In addition, the poultry blocks found around the grazing field located outside the pen did not have fly proofs and were probable source of stable flies. Hogsette and Ose [20] reported high catches inside the periphery of the zoological parks with no statistically significant difference with catches inside and outside. According to Hogsette [20] best catches of stable flies are made near animals, but Murchie [19] realised that stable flies were collected with sticky traps placed at the periphery of a cattle pen. The blue/black colour of the traps used in this trial simply mimic the natural forest edges where stable flies rest and digest their blood meal as well as the two colours reflect light in the UV range [4,21].
Despite the use of insecticide in fly control in most areas in Africa, the obvious shortcoming remains the development of insecticide resistance [7] and its toxic effect on non-target population such as honey bees. An alternative of insecticide usage for fly control is the use of parasitoids [2] and traps that are environmentally friendly [9]. Some of the most common traps used for the control of important pasture flies like tsetse, tabanids and Stomoxyini, include Nzi, Vavoua and Biconical and the Vavoua that has been reported to be highly specific to stable flies [9-11]. However, the Vavoua (10 to 12 Euros) is expensive, difficult to reproduce and not easily available to local farmers and fly control authorities. The modified Vavoua trap (MVT) is a descendant of the Vavoua and is cheaper (7 Euros), simple, easily reproducible, highly specific and available to local farmers of Adamawa through a farmer association (Production Laitiere et Embouche Bovine, Ngaoundere, Cameroon). The Nzi trap is most expensive (14 to 16 Euros) of all the tsetse traps and difficult to transport in the field, but is very expensive. The results of this study indicate that the efficicacy and specificity of the MVT to Stomoxys calcitrans and Stomoxys niger niger was similar to that of the Nzi which is a standard trap. The MVT was unable to catch tabanids that were caught by the Nzi even though with scanty catch. We cannot say that the MVT cannot catch tabanids because the trial was conducted in August and September, characterised by heavy rains and weak abundance of tabanids in Ngaoundere. However, we need to conduct a longitudinal prospection with the MVT, original Vavoua and Nzi in different bioclimatic regions in order to validate this new tool.
We designed a trap known as the modified Vavoua trap (MVT) which is a descendant of the Vavoua trap, it is cheaper and easily reproducible than the Vavoua and Nzi and is available to farmers of the Adamawa region. The MVT recorded similar results in terms of specificity and efficiency as Nzi. The MVT and Nzi traps caught more biting muscids outside the pen as compared to inside. This trap is important because it can reduce the population of stable flies as well as act as a re-infestation barrier for biting fly free rangelands.
The study was successful thanks to the field assistance of Mr Aboubakary (Veterinary technician) and Miss Kong Anita (DVM student). The study was successful thanks to the BWSREK grant received by the first author. Authors acknowledge the technical support of the Laboratory of Vector Ecology (LEV) of the Institute of Research in Tropical Ecology (IRET) in Gabon.
Citation: Lendzele SS, Roland ZKC, Armel KA, Rodrigue MN, Abdoulmoumini M, Lydie AYG, et al. (2020) Efficacy of Modified Vavoua and Nzi Traps in the Capture of Stable Flies: A Preliminary Field Trial in Cameroon. Entomol Ornithol Herpetol.9:230. DOI: 10.35248/2161-0983.20.9.230.
Received: 24-Jul-2020 Accepted: 10-Aug-2020 Published: 17-Aug-2020 , DOI: 10.35248/2161-0983.20.9.230
Copyright: © 2020 Lendzele SS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.