Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2019)Volume 10, Issue 4
Background: Topical retinoid and antibacterial agents are commonly used as first-line treatments in mild/moderate acne vulgaris (AV). Adherence to retinoid treatment could be limited by the low local tolerability. Literature data show that up to 15% of patients prematurely stop the treatment with retinoids because of skin irritation. A medical device in gel formulation containing tretinoin (0.02%), glycolic acid (4%) and clindamycin (0.8%) is available. So far, no real-life, prospective data regarding efficacy and tolerability of this gel are available. We performed a prospective, multicenter, pragmatic 12-week, assessor-blinded trial. Primary outcomes were the changes of Total acne lesions (TL) count. Secondary outcomes were the evolution of Global Acne Grading System score (GAGS) e and Noninflammatory (NI-L) and Inflammatory (I-L) lesions count. An additional secondary outcome was also to evaluate the local tolerability of the gel. Materials and methods: In a real-life multicenter trial, 159 subjects with mild/moderate AV of the face were enrolled after their informed consent in a 12-week trial. Subjects were instructed to apply the gel once daily at evening. Baseline GAGS score was calculated, and a baseline picture of the face was also taken. Visits were performed after 6 and 12 weeks. Clinical evaluation and pictures were performed at the same time-points. GAGS score, NI-L and I-L calculation were performed evaluating subjects’ pictures coded in a randomized fashion by an assessor unaware of time-sequence. At week 6 and 12, an Investigator Global Assessment (IGA) score, using a 5-point scale was also calculated. Outcomes evaluation was performed on an Intention-to-treat basis. Results: A total of 134 (84%) subjects concluded the 12-week treatment period. Twenty-five subjects (16%) concluded prematurely the trial due to poor local tolerability. At baseline the total number of TL was 17 ± 10. Active treatment reduced significantly TL to 11 ± 7 after 6 weeks and to 6.3 ± 5 after 12 weeks, representing a -63% reduction in comparison with baseline. The mean ± SD GAGS score at baseline was 17.3 ± 8. GAGS score was reduced significantly to 11.8 ± 4 after 6 weeks and to 6.3 ± 3 at the end of treatment (a -63% reduction). Both NI-L and I-L lesions numbers were significant reduced at week 6 (NI-L: -42%; I-L:-35%) and at week 12 (NI-L:-65%; I-L:-65%). Tolerability was evaluated good/very good in 123 out of 134 subjects (91%). Conclusion: This gel containing tretinoin, glycolic and clindamycin, used as monotherapy in the treatment of mild to moderate acne, has shown to be effective in reducing TL, NI-L, I-L and GAGS score. The local tolerability profile is in line with other topical retinoids products.
Acne; Topical retinoid; Clindamycin; Prospective trial
Acne is a common inflammatory skin disease affecting sebaceous gland rich areas such as face, thorax and the back [1]. Acne, according with the severity, is characterized by papular, pustular and/or nodulocystic skin eruptions [2]. Acne is more common in teenage years, even if some cases persist also into adulthood [3]. Topical retinoid and antibacterial agents are commonly used as first-line treatments in mild/moderate acne vulgaris (AV) [4,5]. Among retinoids, topical tretinoin is commonly used for its comedolytic and anti-inflammatory action [6]. Clindamycin is a bacteriostatic antibiotic widely used in acne treatment alone or in combination [7]. Clindamycin is very active against Cutibacterium acnes (formerly named Propionibacterium acnes) [8]. In addition, clindamycin has also shown to improve skin tolerability when used with topical retinoid therapy [9]. Glycolic acid is a keratolytic agent which in acne could exert favorable effects normalizing the excessive abnormal desquamation of sebaceous epithelium [10]. Adherence to retinoid or keratolytic treatments could be limited by the low local tolerability [11]. Literature data show that up to 15% of patients stop the treatment with retinoids because of skin irritation [12]. A medical device in gel formulation containing tretinoin (0.02%), glycolic acid (4%) and clindamycin (0.8%) is commercially available. So far, no real-life, prospective, large clinical data regarding efficacy and tolerability of this gel are available. We performed a prospective multicenter, 12-week, assessor-blinded, pragmatic trial in 159 subjects with acne. Study efficacy outcomes were the evolutions of Global Acne Grading System (GAGS) score and Non-inflammatory (NIL), Inflammatory (I-L) and Total (TL) lesions count. Secondary outcome was to evaluate local tolerability.
To evaluate the efficacy and tolerability of a fixed combination of an anti-acne gel based on polyvinyl alcohol (PVA) as excipient and containing tretinoin, clindamycin and glycolic acid in a reallife clinical setting in subjects with mild or moderate/severe acne vulgaris.
We performed a prospective multicenter, 12-week, assessorblinded, pragmatic trial. Primary efficacy outcomes were the evolutions of Global Acne Grading score and Noninflammatory (NI-L) and Inflammatory (I-L) lesions count. Secondary outcome was to evaluate local tolerability.
Study design
This was a multicenter, open, prospective, pragmatic, assessorblinded, 12-week trial. A total of 159 subjects with mild/ moderate AV of the face were enrolled. Written informed consent was taken from all the participating subjects or guardians (for patients below 18 years of age). Informed consent form contained all the relevant information regarding the type of treatment including efficacy and side effects. Study protocol was approved by an independent Investigator Review Board in April 2018. The study was conducted in 15 out-patient primary or secondary Healthcare Dermatology Services in Italy, between June 2018 and May 2019.
Subjects
Eligible subjects were adult men or women aged 15 or over with a clinical diagnosis of mild-to-moderate AV (less than 30 total lesions). Exclusion criteria were the presence of severe AV, recent (less than 4 weeks) topical or oral anti-acne treatments (like antibiotics and retinoids), the use of oral contraceptive in the previous 3 months pregnancy or breast feeding, a Body Mass Index of ≥ 25 and a known history of allergy to one of the components of the tested product. Subjects were instructed to apply the gel once daily in the evening after facial cleansing. The amount of product for each application was 1 Finger-Tip-Unit (0.5 g), on average. The subjects were instructed to use as common daily skin care only emollients and non-aggressive cleansers Visits were performed at baseline and after 6 and 12 weeks.
Study outcomes
The primary study outcome was:
• The change of total AV lesion count (TL; non-inflammatory plus inflammatory lesions) calculated in blinded manner evaluating high definition digital standardized (distance and light conditions) colored subjects ’ pictures, coded in a randomized fashion, by an assessor unaware of time-sequence. Smart-phone selfie pictures of good quality were also allowed as clinical documentation. All the subjects gave the permission for the use of pictures for the publication.
Secondary endpoints of the trial were:
• The evolution of baseline Global Acne Grading System (GAGS score) according to Doshi et al. [13] calculated at baseline and after 6 and 12 weeks. In more details, GAGS divides the face, chest, and back into six areas (forehead, each cheek, nose, chin and chest, and back) and assigns a factor to each area by size. Each type of lesion is given a value depending on severity: no lesions=0, comedones=1, papules=2, pustules=3, and nodules=4. The score for each area (local score) is calculated using the following formula: local score=Factor 9 Grade (0-4). The global score is the sum of local scores. Acne severity was graded using the obtained global score for the areas included (forehead, cheeks, nose, and chin).
• The Investigator Global Assessment (IGA) score, using a 5- point score scale (from -1, worsening; to +3, great improvement with complete clearing of acne lesions) calculated at week 6 and week 12.
• The Investigator Tolerability Assessment (ITA) score using a 4- point scale (from 0: very low tolerability to 3: very good tolerability) evaluated at week 6 and week 12. The local tolerability was also evaluated checking patient’s self-reported side effects.
Primary and secondary outcomes evaluation was performed on an Intention-to-treat (ITT) basis using the Last-Observation- Carried-Forward (LOCF) method. Safety and skin tolerability were assessed recording any spontaneous side effect reported by the subject at each visit. No specific evaluation of compliance to the treatment was performed.
Statistical analysis
Statistical analysis was performed using GraphPad statistical software (GraphPad Software, Inc. La Jolla, CA, USA). Continuous variables were expressed as mean ± standard deviation (SD). The primary endpoint of the trial was the evolution of total AV lesion count (non-inflammatory, NI-L, inflammatory, I-L and total, TL) from baseline and after 6 and 12 weeks of treatment. The paired t test and the Wilcoxon test were used for the evaluation of the variables during the study (baseline, day 30 and day 60). For the primary outcome variables, we calculated also the 95% confidence intervals to give an estimation of the effect size and its precision. Analysis was performed on the basis of intention-to-treat (ITT) principle. A value of p ≤ 0.05 was considered statistically significant. A formal sample size calculation was not performed. We decided to enroll at least 130 subjects.
A total of 134 (84%) subjects concluded the 12-week treatment period. Subjects ’ characteristics are presented in Table 1. Twenty-five subjects (16%) concluded prematurely the trial due to poor local tolerability. At baseline the total number of lesions (non-inflammatory, N-IL plus inflammatory, IL, lesions) was 17 ± 10. Active treatment reduced significantly this number to 11 ± 7 after 6 weeks and to 6.3 ± 5 after 12 weeks, representing a -63% reduction in comparison with baseline (Figure 1). At week 12 the absolute difference in total lesion number in comparison with baseline was -11.3 (95% CI: from -9.9 to -12.7) Both NI-L and I-L lesions numbers were significant reduced at week 6 (NIL: -42%; I-L: -35%) and at week 12 (NI-L:-65%; I-L:-65%) (Figure 2). At baseline, the mean ± SD GAGS score was 17 ± 8, confirming the enrollment, according with inclusion criteria, of subjects with mild or mild-moderate acne. GAGS score was reduced significantly to 11.8 ± 4 after 6 weeks and to 6.7 ± 3 at the end of treatment (a -61% reduction) with an absolute reduction of -10.3 (95% CI: from -8.7 to -11.9). The IGA score at week 6 was 1.3 and 2.0 at week 12 (Figure 3). The percentage of subjects with an IGA score of 2 or 3 (clear or almost clear), at week 12, was 68% (109 out of 159, ITT) (95% CI from 61% to 75%). Tolerability was evaluated good/very good (ITA score of 2 or 3) in 123 out of 134 subjects (91% per protocol or 77% in ITT analysis). Figures 4-9 report some clinical pictures of treated subjects at baseline and after 12 weeks of treatments.
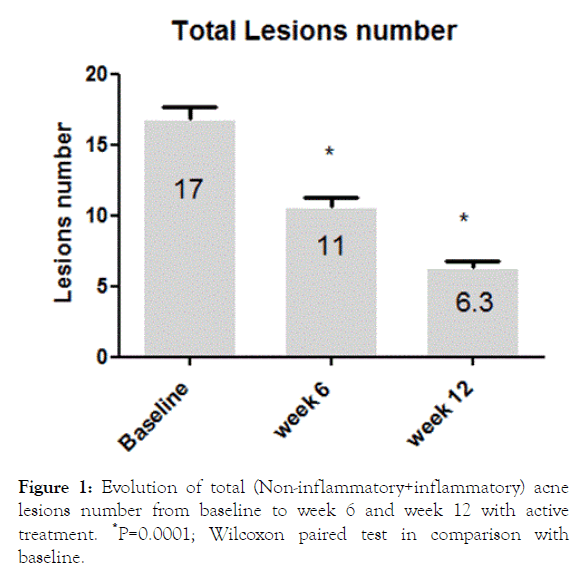
Figure 1. Evolution of total (Non-inflammatory+inflammatory) acne lesions number from baseline to week 6 and week 12 with active treatment. *P=0.0001; Wilcoxon paired test in comparison with baseline.
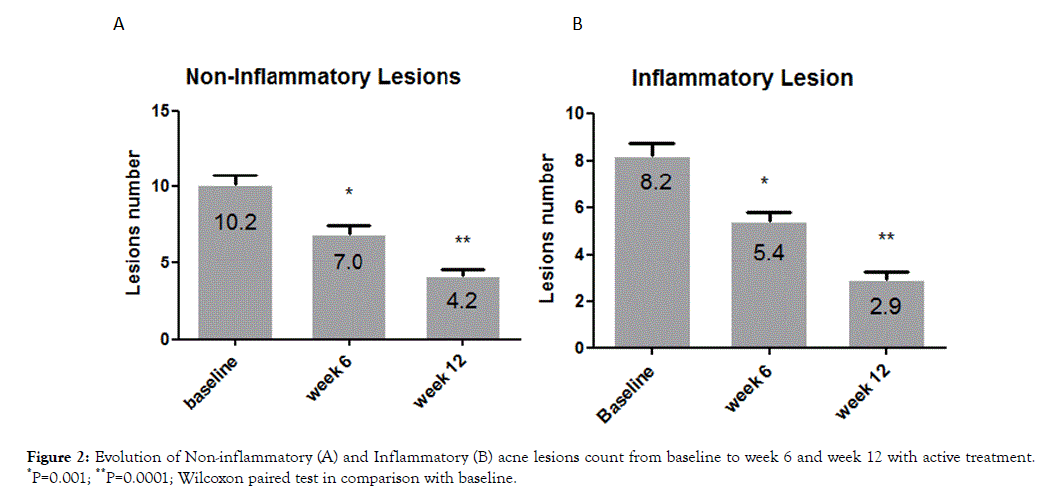
Figure 2. Evolution of Non-inflammatory (A) and Inflammatory (B) acne lesions count from baseline to week 6 and week 12 with active treatment. *P=0.001; **P=0.0001; Wilcoxon paired test in comparison with baseline.
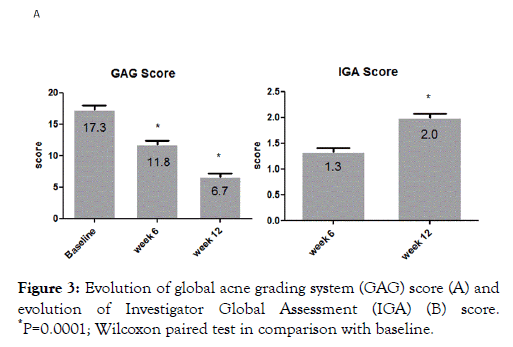
Figure 3. Evolution of global acne grading system (GAG) score (A) and evolution of Investigator Global Assessment (IGA) (B) score. *P=0.0001; Wilcoxon paired test in comparison with baseline.
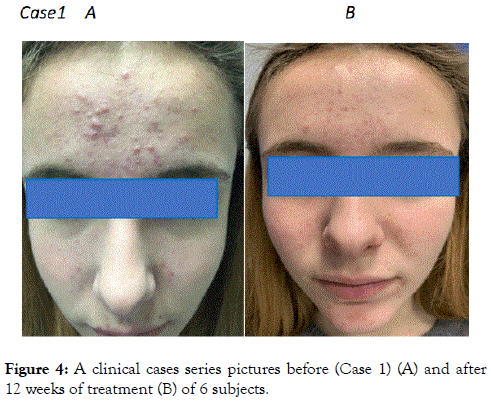
Figure 4. A clinical cases series pictures before (Case 1) (A) and after 12 weeks of treatment (B) of 6 subjects.
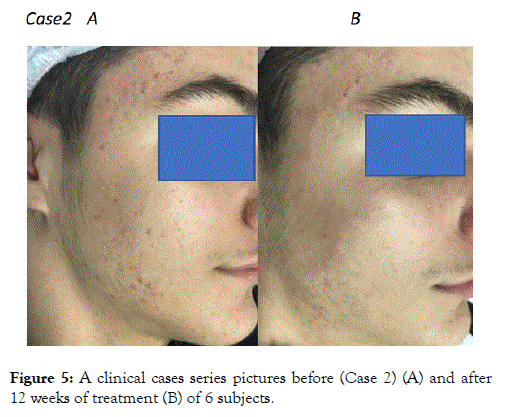
Figure 5. A clinical cases series pictures before (Case 2) (A) and after 12 weeks of treatment (B) of 6 subjects.
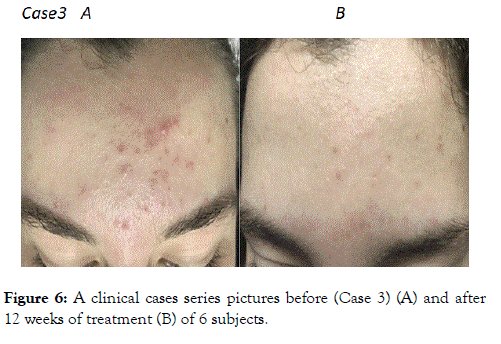
Figure 6. A clinical cases series pictures before (Case 3) (A) and after 12 weeks of treatment (B) of 6 subjects.
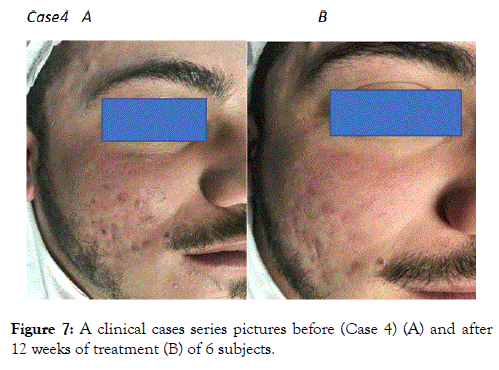
Figure 7. A clinical cases series pictures before (Case 4) (A) and after 12 weeks of treatment (B) of 6 subjects.
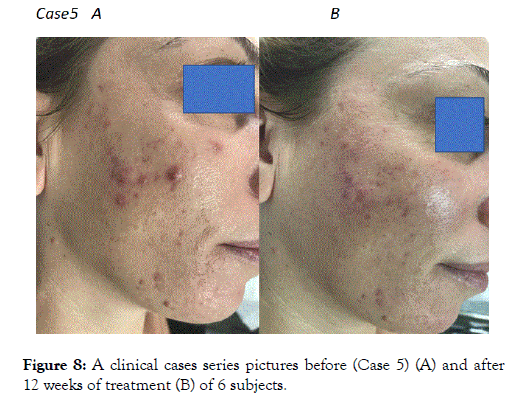
Figure 8. A clinical cases series pictures before (Case 5) (A) and after 12 weeks of treatment (B) of 6 subjects.
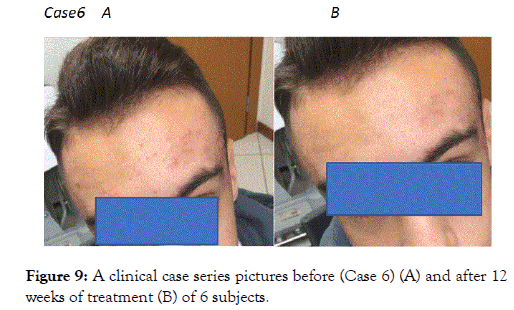
Figure 9. FigureA clinical case series pictures before (Case 6) (A) and after 12 weeks of treatment (B) of 6 subjects.
| Enrolled Subjects | 159 |
|---|---|
| Drop out; n (%) | 25 (16) |
| Subjects completing the trial, n (%) | 134 (85) |
| Men/Women; n (%) | 68/91 (43%/57%) |
| Mean age, years (mean ± SD) | 20 ± 5 |
| Body Mass Index | 21 ± 5 |
| Skin Photo type | |
| I | 0.025 |
| II | 0.52 |
| III | 0.37 |
| IV | 8.5 |
| Total acne Lesions (mean ± SD) | 17 ± 10 |
| GAGS Score at baseline | 17.3 ± 8 |
Table 1: Subjects characteristics at baseline.
Acne is a very common inflammatory skin disease affecting the pilosebaceous gland unit [13,14]. The main factors promoting acne lesions are plugged follicles, increased sebum production and Cutibacterium acnes induced skin inflammation [15]. Acne lesions are commonly divided in non-inflammatory (open and closed comedones) and inflammatory lesions (papules, pustoles and nodules) [16]. Acne is a multifactorial skin disease and therefore a therapeutic approach using different active principles is often necessary. Topical retinoid/antimicrobial combination therapies are considered as first line treatment for patients with moderate/moderate-severe acne [17]. In our trial, we evaluated the efficacy and tolerability of a peculiar fixed combination of a retinoid (tretinoin), an antibiotic (clindamicin) and a keratolytic agent (glycolic acid). Retinoids can reduce the formation of microcomedones, normalizing keratinocytes desquamation [18]. In addition, these molecules could have anti-inflammatory effects [19]. Clindamycin targets Cutibacterium acnes and has also an anti-inflammatory activity [20]. Finally, the third component of the tested product is glycolic acid which is a keratolytic agent which in acne could exert favorable effects normalizing the excessive abnormal desquamation of sebaceous epithelium. Glycolic acid could also enhance the penetration of the two other components of the gel [21]. Our study shows that in subjects with moderate/moderately severe acne the 12-weeks application of this three-component gel is effective with a -63% of reduction in total number of acne lesions after 12 weeks of treatment. This reduction is at least comparable with the efficacy data available for a similar topical product containing a combination of clindamycin and tretinoin [22]. For the latter product, a metanalysis of three trials has shown a reduction of total lesion number of -52.5%. Tretinoin monotherapy reduced total acne lesions by -50% at week 12 [23]. The tolerability profile of the gel evaluated in our study seems comparable to the data available for other topical retinoid products. In the present study 25 subjects (16%) dropped out prematurely due to skin irritation. This percentage is comparable to published data. In fact, Veraldi et al. [12] reported that 15% of tretinoin treated subjects stop prematurely the use of the product because skin irritation. Some limitations should be taken in account in evaluating our results. First, we have performed an open noncontrolled trial. To increase the internal validity of the trial results, we decided to adopt an assessor-blinded approach in evaluating the main clinical efficacy outcomes of the study (evolution of acne lesions and the evolution of GAGS score). On the other hand, one strength of our trial however is related with the pragmatic nature of the study, carried out in real life condition. These aspects increase the external validity of the results we have found. In the present study there was not a control group or a vehicle treated group. However the clinical efficacy we observed could hardly ascribed to the excipient of the tested gel (PVA) only, in consideration of the range in the reduction of acne lesion we observed.
A gel containing tretinoin, glycolic and clindamycin, used as monotherapy in the treatment of mild to moderate acne, has shown to be effective in reducing GAGS score and TL, NI-L and I-L. The clinical efficacy was comparable to other antibiotic/ retinoid topical products. The local tolerability profile is in line with other topical retinoids products.
MM is an employee of Cantabria Labs Difa Cooper; a pharma company, producing and marketing the tested product (Mask Plus™, Cantabria Labs Difa Cooper). MM participated to the study protocol preparation and was involved in the final version of the manuscript. MM was not involved in data analysis and statistical evaluation. All the other MPlus investigators were involved in the conduction of the trial (selection of subjects, visits, medical evaluation, data collection, picture coding and blinded evaluation and data analysis). All the MPlus Investigators declare no conflicts of interest.
Citation: Milani M, Cortelazzi C, Chieco P, Ferrazzi A, Gargano N, Maiani E, et al. (2019) Efficacy and Tolerability of a Tretinoin 0.02%, Clindamycin 0.8% and Glycolic Acid 4% Gel in Acne: A Multicenter, Prospective, Pragmatic, Assessor-Blinded, 12-Week Trial on 159 Subjects. J Clin Exp Dermatol Res. doi: 10.35248/2155-9554.10.503
Received: 27-Jul-2019 Accepted: 13-Aug-2019 Published: 21-Aug-2019 , DOI: 10.35248/2155-9554.19.10.503
Copyright: ©2019 Milani M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.