Endocrinology & Metabolic Syndrome
Open Access
ISSN: 2161-1017
ISSN: 2161-1017
Research Article - (2022)Volume 11, Issue 4
Background and aim: According to TCM theory, the kidney-yang is the basis of human functional activities, and the elderly will appear insufficient kidney-yang. Moxibustion is an effective treatment for KYDS, but the exact mechanism remains to be fully elucidated. This research aims to investigate the effects of moxibustion on the hippocampal-HPA axis in aged rats and to elucidate the possible molecular mechanisms.
Method: Thirty-six old male rats were randomly divided into Old Rats Control Group (ORC), moxibustion group (moxi) and Carbenoxolone Group (CBX), and 12 adult rats were included in the young rats control group (YRC), with 12 rats in each group. Rats in the moxi group were received moxibustion and rats in the CBX group were received CBX injection. After treatment, the serum levels of CORT, ACTH and CRH, and the expressions of MR, GR and 11β-HSD1 in rats’ hippocampus were assessed.
Results and conclusion: Before treatment, the serum level of CRH, ACTH and CORT, and the expressions of MR, GR and 11β-HSD1 in hippocampus of rats in the ORC group were all significant lower than those of rats in the YRC group (P<0.01). After treatment with moxibustion, all the afore mentioned observation indices of rats were significant higher than those of rats in the ORC group (P<0.05 or 0.01). Moxibustion can improve the serum levels of ACTH, CRH and CORT, and can up-regulate mRNA and protein expressions of MR, GR and 11β-HSD1 in the hippocampus of aged rats.
Moxibustion; Kidney deficiency; Mineralocorticoid receptor; Glucocorticoid receptor; 11 betahydroxysteroid dehydrogenase type 1
A condition associated with aging is called frailty syndrome [1]. It is common in the elderly and associated with a functional multiple organ system declines. It is often associated with various metabolic disorders such as changes in energy metabolism in muscle, bone and the endocrine system [2]. Early diagnosis and treatment of frailty can significantly promote healthy aging [2]. According to the theory of traditional Chinese medicine, the kidney-yang is the basis of human functional activities, and the elderly will appear insufficient kidneyyang. This results in symptoms that include cold limbs, fear of cold, frequent urination, and night urination that is extended and clear. Recent studies have shown that the main basis for Kidney-Yang Deficiency Syndrome (KYDS) is a disorder of the Hypothalamic Pituitary Adrenal axis (HPA axis) [3]. Metabolic abnormalities associated with old age frequently involve the HPA axis [2]. Therefore, application of traditional Chinese medicine is a useful way to promote the recovery of the kidney yang as it applies to the HPA axis. This may allow further exploration of mechanisms involving the kidney yang and its role in regulating metabolism in the elderly thereby providing effective treatment methods for dealing with associated disorders.
The HPA axis is affected both through negative feedback regulation of hormones secreted by each organ and by the regulation of the hippocampus, the upper control center. Glucocorticoid (GC) regulation is a key role for the HPA axis as well as that of the hippocampus-HPA axis and the associated stress responses [4]. Mineralocorticoid Receptors (MRs) and Glucocorticoid Receptors (GRs) in the hippocampus participate in regulation of the HPA axis by binding GC [5]. The negative feedback regulating effects of GC on target regions, including the HPA axis, depend upon levels of GR and MR in the hippocampus and GC concentrations in the target areas. In addition to being influenced by the concentration of free GC in the plasma, levels of GC in the target regions are mainly regulated by the GC metabolic enzyme, 11beta-hydroxysteroid dehydrogenase (11β-HSD) [6-8]. 11β-HSD 1 affects the binding of MR, GR and GC in the hippocampus by pre-receptor regulation and indirectly affects the normal function of the hippocampus.
KYDS is an important syndrome in Traditional Chinese Medicine (TCM). As described in the introduction to medicine, a classic TCM scholar work, treating KYDS deficiency syndrome in the elderly involves the use of moxibustion. Moxibustion is a traditional type of Chinese medicine therapy that uses a preparation of burning dried mugwort (moxa). It is widely used in China for the treatment of various chronic diseases and deficiencies [8]. Suspended moxibustion is an indirect form of moxibustion in which the dried mugwort is placed over an acupoint without skin contact to stimulate the circulation by warming the acupoint and improving the flow of blood and Qi. This facilitates health and recovery from disease [8]. Research and clinical observations indicate that moxibustion therapy is an effective treatment for KYDS, but the exact mechanism remains to be fully elucidated [9-12].
Our previous research has found that suspended moxibustion treatment at the Shenshu and Guanyuan acupoints with a moxa stick can effectively improve dysfunction of the HPA axis [13]. Thus, we hypothesized that a possible mechanism by which moxibustion improves KYDS in aged rats may be by up-regulating mRNA and protein expression of MR, GR, and 11β-HSD1 in the hippocampus- HPA axis. This, in turn, would improve serum levels of ACTH, CRH and CORT. The purpose of this study was to further elucidate the molecular mechanisms by which moxibustion can be of be of benefit when treating KYDS in aged rats.
Experimental animals
Thirty six male, Sprague Dawley (SD) rats aged (22-month-old, 680 ± 20 g) and 12 male, SD rats aged 5 months old (weighing 720 ± 20 g ) were purchased from the Experimental Animal Center of Jiangxi University of Traditional Chinese Medicine (Jiangxi, China, certificate no. JZLLSC (jiangxi) 2017-121). The rats had free access to standard rat chow and water in a controlled environment at constant temperature (20℃-25℃) on a 12/12 h light/dark cycle. All procedures were conducted in accordance with guidelines reviewed and approved by the Institutional Animal Care and Use Committee of Jiangxi University of Traditional Chinese Medicine, China.
Treatment groups
The 36 month old aged rats were randomly divided into an Old Rat Control Group (ORC, n=12), a moxibustion group (moxi, n=12) and a Carbenoxolone Group (CBX, n=12). In addition, 12 young adults (5 months of age) formed the Young Rat Control group (YRC, n=12).
In the moxi group, double accupoints guanyuan (CV4) and shenshu (BL23) were selected and locating based on experimental acupuncture guidelines [14]. Before treatment, the treatment sites were shaved at the revelant acupoints to expose bare skin. The acupoints guanyuan (CV4) and shenshu (BL23) were exposed to heat using a suspended moxibustion paper tube containing burning mugwort (length 12 cm, diameter 0.6 cm; custom made for use with animals in the Affiliated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang, China). The moxibustion tube was suspended at a height of approximately 3 cm over the hairless areas of the skin for 20 min every other day for 2 months. Each rat was restrained on a wooden platform during moxibustion treatment.
Rats in the CBX group received no moxibustion treatment and instead received intraperitoneal injections of carbenoxolone (10 mg/(kg.d)), every other day for 2 months [15]. Each rat was restrained in the same manner as the moxi group every other day for 2 months.
Rats in the ORC group and the YRC group were restrained without moxibustion and received intraperitoneal injections of saline (10 mg/ (kg.d)) every other day for 2 months.
Sample collection
The experimental procedure was carried out as described previously with minor modifications. Before and after treatment, the rats were placed in the behavior test room for a 24 h adaptation period [16]. Each test took place in an empty box (60 cm × 60 cm × 50 cm) [16]. Rats in the empty box were allowed to explore freely for 10 min after which two objects labeled A and B, which were of identical size, shape, color and texture, were simultaneously placed in the test box. Rats in the test box were allowed to explore the objects freely for 10 min. After this period the rats were removed from the box and one of the objects was replaced by a third novel object C that was of a completely different size, shape, color and texture. The rats were returned to the box and allowed to continue exploring for another 10 minutes. Total time spent exploring each object (sniffing, learning, and climbing) was recorded. For each rat, novel object interest and activity level was measured using the novel objects interest index which is equal to the time spent exploring object C/the total time × 100%.
In addition, grooming, defecation, urination, and other behaviors were noted. A dark, quiet test environment was maintained to reduce distraction caused by the external environment. The test arena floor and the inner wall were cleaned thoroughly before and after each test with a moist cloth, then with 90% ethanol to remove any residue of odorants and marks left by the previous test subjects.
Enzyme Linked Immunosorbent Assay (ELISA)
Urinary 17-OHCS and the serum levels of testosterone, estradiol, CORT, ACTH and CRH were determined using an ELISA kit purchased from Shanghai Bogu Biotechnology Co., LTD (Shanghai, China). The ELISA test was conducted according to protocol provided by manufacturer.
Reverse transcription Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
RT-qPCR was performed as described previously with minor modifications [17]. Total RNA was isolated from the hippocampus of rats in each group using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA). RNA concentration and purity were evaluated by spectrophotometry (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was synthesized from the extracted RNA using a random primer and RevertAid™ M-MuLV reverse transcriptase (Fermentas), according to the manufacturer's instructions. cDNAs were amplified by PCR using primers for each target gene. The mRNA expression levels of MR, GR and 11β-HSD1 were measured using an RT-qPCR system with SYBR Green (Thermo Fisher Scientific, Waltham, MA, USA). PCR was performed in a final volume of 25 μl, containing 12.5 μl 2 × PCR master mix, 2 μl cDNA, 1 μl forward primer, 1 μl reverse primer, and 8.5 μl sterilized DEPC water. RT-qPCR conditions were as follows: 94°C for 5 minutes, followed by 40 cycles at 95°C for 15 seconds, 60°C for 45 seconds and 72°C for 30 seconds. The fluorescence signal was detected at 60°C, and the samples were finally extended at 72°C for 7 minutes. The amplification efficiency was compared between the target and a reference control Glyceraldehyde 3 Phosphate Dehydrogenase (GAPDH) using the delta-delta Ct (ΔΔCt) method [13]. The primers employed are shown in Table 1.
| Gene | Full name | Sequences (5–3′) | Product size (bp) |
|---|---|---|---|
| MR | Mineralocorticoid Receptor | Sense primer: AGA AGC TGG GGA AGT TAA AAG G antisense primer: TCG GAG CGA TGT ATG TGG TC | 102 |
| GR | Glucocorticoid Receptor | Sense primer: CAT TAC CAC AGC TCA CCC CTA C antisense primer: GCA ATC ACT TGA CGC CCA C | 148 |
| 11β-HSD1 | 11 beta- Hydroxysteroid Dehydrogenase type 1 | Sense primer: AAA ATA CCT CCT CCC CGT CC antisense primer: AGG CAG CGA GAC ACC ACC | 219 |
| GAPDH | Glyceraldehyde 3-Phosphate Dehydrogenase | Sense primer: GGA GTC TAC TGG CGT CTT CAC antisense primer: ATG AGC CCT TCC ACG ATG C | 237 |
Table 1: Primer sequences for real-time quantitative polymerase chain reaction.
Western blot assay
Western blot analysis was performed as described previously with minor modifications [18,19]. All hippocampus tissues obtained in each group were homogenized in RIPA lysis buffer with protease inhibitor (JRDUN Biotechnology, Shanghai, China). The samples were centrifuged at 12,000 r/min for 20 minutes at 4°C and an aliquot of the supernatant was taken to determine protein levels using the BCA assay. Equal amounts of proteins were separated by Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and then the resolved proteins were transferred to Polyvinylidene Fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were incubated with primary antibodies overnight at 4°C. The antibodies used for Western blotting included rat monoclonal antibodies anti-MR (1:1000), anti-GR (1:1000), anti-11β-HSD1 (1:1000) and anti-GAPDH (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The membranes were washed 5 minutes with Tris-buffered saline Tween 3 times and incubated with goat anti-rabbit IgG-HRP (1:1000; Beyotime Biotechnology, Shanghai, China) and goat anti-mouse IgG-HRP PS1 (C-20) (1:1000; Beyotime Biotechnology, Shanghai, China) antibodies at 37°C for 1 hour. The immunoreactive bands were visualized using an enhanced chemiluminescence reagent (Beyotime Biotechnology, Shanghai, China). Grayscale values of the bands were quantified using image j software (Fujifilm, Tokyo, Japan). The relative expression of protein was calculated based on the ratio of bands values to loading control.
In situ hybridization
In situ hybridization was performed as described previously with minor modifications [20]. Six paraffin blocks were randomly selected and cooled to -20℃ for at least 30 minutes. The blocks were then cut into 4-7 μm-thick serial sections. After being de-paraffinized and dehydrated, each section was incubated in 50 μl hybridization buffer containing a 10 µM oligonucleotide probe at 95℃ for 5 minutes and 37℃-40℃ for 12 hours, washed three times with 5X, 1X and 0.2X saline sodium citrate, and treated with blocking buffer at 37℃ for 15 minutes. The blocking buffer was then blotted with paper. Each section was incubated in 30 µl biotinylated anti-digoxin antibody (1:50) at 37℃ for 1 hour, washed four times with 0.5 M PBS, incubated in streptavidin-biotin-peroxidase complex at 37℃ for 30 minutes, and rinsed four times in 0.5 M PBS. Subsequently, the sections were developed in 3, 3, diaminobenzidine, counterstained with hematoxylin, dehydrated in alcohol, permeabilized in xylene, mounted with proof quench mounting agent, and photographed with a fluorescence microscope. Upper, middle, lower, left and right visual fields were randomly selected for each section and image-pro Plus 6.0 software was used to detect the integrated optical density of positive cells after which the average value was used for statistical analysis. Negative controls were incubated in 0.01 M PBS without primary antibody. The probes employed are shown in Table 2.
| Gene | Full name | Sequences (5–3′) |
|---|---|---|
| MR | Mineralocorticoid Receptor | GGAUGGAGAGGAUAGCAAUCCCGGCAGUCGCCCUACUGACGGUGGG |
| GR | Glucocorticoid Receptor | UGCUUGUGGAGCCUUUCGAGAAAUCAAGGAGAAUCCUCUGCUGCUU |
Table 2: Primer sequences for Hybridization in situ.
Statistical analyses
All data are presented as mean ± SD. Data were analyzed using oneway ANOVA followed by a post hoc Student-Newman-Keuls (SNK) test using SPSS 19.0 software (SPSS, Chicago, IL, USA). A value of P<0.05 was considered statistically significant.
Evaluation of rats’ status
General condition observations: In the YRC group, the rats appeared to be in good condition with smooth and shiny hair, active and interested in novel objects in their surroundings. By contrast, the rats in the ORC group were less active, less responsive, less interested in novel things, displayed an arched back, clustered together in groups, and their hair had lost its luster. After treatment with moxibustion, the ORC rats’ activity level plus interest in novel things improved and their hair gradually became more shiny. The general condition of the rats in the CBX group improved to a similar degree compared to rats in the moxi group.
Interest index and urinary level of 17-OHCS: Compared with those in the YRC group, the interest index of rats in the ORC group was significantly lower (P<0.01). After treatment with moxibustion, the interest index of the rats was significant higher than that of rats in the ORC group (P<0.01). The interest index of rats in the CBX group was significant lower than that of rats in the moxi group (P<0.01). In the moxi group, the interest index of rats was increased significantly after treatment compared to before treatment values (P<0. 01, Figures 1A and 1B).
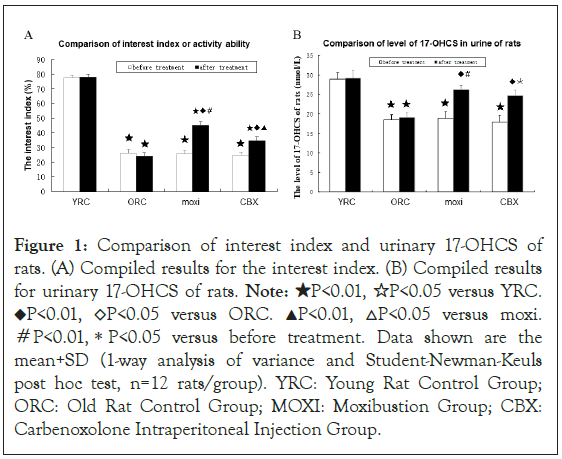
Figure 1: Comparison of interest index and urinary 17-OHCS of rats. (A) Compiled results for the interest index. (B) Compiled results for urinary 17-OHCS of rats. Note: ★P<0.01, ☆P<0.05 versus YRC. ◆P<0.01, ◇P<0.05 versus ORC. ▲P<0.01, △P<0.05 versus moxi. #P<0.01,*P<0.05 versus before treatment. Data shown are the mean+SD (1-way analysis of variance and Student-Newman-Keuls post hoc test, n=12 rats/group). YRC: Young Rat Control Group; ORC: Old Rat Control Group; MOXI: Moxibustion Group; CBX: Carbenoxolone Intraperitoneal Injection Group.
Compared with those in the YRC group, the level of urinary 17-OHCS of rats in the ORC group was significantly decreased (P<0.01). After treatment with moxibustion, the level of urinary 17-OHCS of rats was significantly higher than that of rats in the ORC group (P<0.01). The level of urinary 17-OHCS of rats in the moxi and CBX group were significant higher after treatment than before respectively (P<0. 01 or 0.05; Figure 1).
Serum levels of testosterone and estradiol: Compared with rats in the YRC group, serum levels of testosterone were significantly decreased while estradiol increased in the ORC group (P<0.01). After treatment with moxibustion, serum levels of testosterone were significantly higher and estradiol was lower than those of rats in the ORC group (P<0.01 or 0. 05). After treatment with CBX, serum levels of testosterone were significantly lower than those of rats in the ORC group (P<0. 05).
Compared with the YRC group, the ratio of testosterone to estradiol was significantly decreased in the ORC group (P<0.01). After treatment with moxibustion or CBX, the ratio of testosterone to estradiol was significantly higher in both groups compared to those in the ORC group (P<0.01). Compared with moxi group, the ratio of testosterone to estradiol was significantly lower than that in the CBX group (P<0.05; Figure 2).
Figure 2: Comparison of testosterone and estradiol levels in serum. Compiled results of (A) testosterone, (B) estradiol, (C) testosterone to estradiol ratio. Note: ★P<0.01, ☆P<0.05 versus YRC. ◆P<0.01, ◇P<0.05 versus ORC. ▲P<0.01, △P<0.05 versus moxi. Data are shown as the mean ± SD (1-way analysis of variance and Student-Newman- Keuls post hoc test, n=12 rats/group). YRC: Young Rat Control Group; ORC: Old Rat Control Group; MOXI: Moxibustion Group; CBX: Carbenoxolone Intraperitoneal Injection Group.
Statistical processing of data was performed with Statistica 6.0. Mann- Whitney U criterion was used for processing of the non-parametric data. Differences were considered significant under p<0.05.
Serum levels of CORT, ACTH and CRH
Compared with those in the YRC group, the serum levels of CORT, ACTH and CRH of ORC decreased significantly (P<0.01). After treatment with moxibustion, serum levels of CORT, ACTH and CRH in the moxi group were significantly higher than those in the ORC group (P<0.01). Compared with those in the moxi group, the serum levels of CORT and ACTH of the CBX group significantly decreased (P<0.01, Figure 3).
Figure 3: Comparison serum CORT, ACTH and CRH. Compiled results for (A) CORT, (B) ACTH, and (C) CRH. Note: ★P<0.01, ☆P<0.05 versus YRC. ◆P<0.01, ◇P<0.05 versus ORC. ▲P<0.01, △P<0.05 versus moxi. Data are shown as the mean ± SD (1-way analysis of variance and Student-Newman-Keuls post hoc test, n=12 rats/group). YRC: Young Rat Control Group; ORC: Old Rat Control Group; MOXI: Moxibustion Group; CBX: Carbenoxolone Intraperitoneal Injection Group; CORT: Corticosterone; ACTH: Adrenocorticotrophic Hormone; CRH: Corticotropin Releasing Hormone.
The subcutaneous administration of the CM total fraction caused a significant decrease in the level of the cytolytic enzyme compared to the control: by 75% in the first 4 hours, and by 50% in the first 24 hours. The subcutaneous administration of>30 kDa fraction resulted in a maximum significant reduction in GOT blood levels at the beginning of the acute period after administration of APAP (85% compared to control).
mRNA expressions of MR, GR and 11β-HSD1 in hippocampus
Expression of MR, GR and 11β-HSD1 mRNA in the hippocampus the ORC group was significantly lower than that of the YRC group (P<0.01). After treatment with moxibustion, MR, GR and 11β-HSD1 mRNA expression in the hippocampus of the moxi group was significantly higher than that of the ORC group (P<0.01). MR, GR and 11-HSD1 mRNA expression in the hippocampus of the CBX group was significantly lower than that of the moxi group (P<0.05 or 0.01, Figure 4).
Figure 4: Comparison of the relative MR, GR and 11β-HSD1 mRNA expression in the hippocampus. Compiled results for the relative mRNA expression of (A) MR, (B) GR, and (C) 11β-HSD1. Note: ★P<0.01, ☆P<0.05 versus YRC. ◆P<0.01, ◇P<0.05 versus ORC. ▲P<0.01, △P<0.05 versus moxi. Data are shown as the mean ± SD (1-way analysis of variance and Student-Newman-Keuls post hoc test, n=6 rats/group). YRC: Young Rat Control Group; ORC: Old Rat Control Group; MOXI: Moxibustion Group; CBX: Carbenoxolone Intraperitoneal Injection Group; MR: Mineralocorticoid Receptor; GR: Glucocorticoid Receptor; 11β-HSD1: 11 beta Hydroxysteroid Dehydrogenase type 1; GAPDH: Glyceraldehyde-3 Phosphate Dehydrogenase.
Western blot analysis of protein expression of MR, GR and 11β-HSD1 in hippocampus
MR, GR and 11β-HSD1 protein expression in the hippocampus of the ORC group was significantly lower than that of the YRC group (P<0.05 or 0.01). After treatment with moxibustion, MR, GR and 11β-HSD1 protein expression in the hippocampus of the moxi group was significantly higher than the ORC group (P<0.05 or 0.01). Except for MR, GR and 11β-HSD1 protein expression in the hippocampus of the CBX group was significantly lower than that of the moxi group (P<0.05 or 0.01, Figure 5).
Figure 5: MR, GR and 11β-HSD1 activation in hippocampus following treatment. (A) Western blots for MR, GR, 11β-HSD1 and GAPDH expression. Compiled results for (B) MR/GAPDH expression, (C) GR/GAPDH expression, and (D) 11β-HSD1/GAPDH expression. Note: ★P<0.01, ☆P<0.05 versus YRC. ◆P<0.01, ◇P<0.05 versus ORC. ▲P<0.01, △P<0.05 versus moxi. Data are shown as the mean ± SD (1-way analysis of variance and Student-Newman-Keuls post hoc test, n=6 rats/ group). YRC: Young Rat Control Group; ORC: Old Rat Control Group; MOXI: Moxibustion Group; CBX: Carbenoxolone Intraperitoneal Injection Group; MR: Mineralocorticoid Receptor; GR: Glucocorticoid Receptor; 11β-HSD1: 11 beta Hydroxysteroid Dehydrogenase type 1; GAPDH: Glyceraldehyde-3 Phosphate Dehydrogenase.
In situ hybridization analysis of mRNA expression of MR and GR in the hippocampus
As shown in Figure 6, in situ hybridization-positive cells exhibited brown spots or particles (arrows). In the YRC group, considerable MR and GR mRNA expression was observed in the CA1 or CA3 of the hippocampus. Minimal mRNA expression of MR and GR was observed in the hippocampus. The integral optical density (IOD) mRNA expression values for MR and GR in the hippocampus of the ORC group was significantly decreased (P<0.01) compared with those in the YRC group. Treatment with moxibustion increased mRNA expression of MR and GR. IOD mRNA expression of MR and GR in the hippocampus of the moxi group was significantly higher than that of the ORC group (P<0.01). IOD MR and GR mRNA expression in the CBX group was significantly lower than that of the moxi group (P<0.01, Figures 6 and 7).
Figure 6: mRNA expression of MR and GR in the hippocampus following treatment (in situ hybridization, X200). Note: Bar=50 µm. YRC: Young Rat Control Group; ORC: Old Rat Control Group; MOXI: Moxibustion Group; CBX: Carbenoxolone Intraperitoneal Injection Group; MR: Mineralocorticoid Receptor; GR: Glucocorticoid Receptor.
Figure 7: Compiled IOD values for MR and GR mRNA-positive cells. Compiled IOD results for (A) MR, and (B) GR. Note: ★P<0.01, ☆P<0.05 versus YRC. ◆P<0.01, ◇P<0.05 versus ORC. ▲P<0.01, △P<0.05 versus moxi. Data are shown as the mean ± SD (1-way analysis of variance and Student-Newman-Keuls post hoc test, n=6 rats/group). YRC: Young Rat Control Group; ORC: Old Rat Control Group; MOXI: Moxibustion Group; CBX: Carbenoxolone Intraperitoneal Injection Group; MR: Mineralocorticoid Receptor; GR: Glucocorticoid Receptor; IOD: Integrated Optical Density.
With an increase in age, organ function in the elderly gradually declines and metabolism decreases as does the level of activity or interest in novel things. In this study, the interest index of aged rats in the ORC group were significantly lower than that of young rats in the YRC group. The level of urinary 17-OHCS of aged rats was significantly lower than that of young rats. Urinary 17 Hydroxycorticosteroids (17-OHCS) are adrenal corticosteroid metabolites and their levels are a measure of adrenal cortex function [3]. Found that the 24 h level of urinary 17- OHCS in patients with kidney-yang deficiency syndrome associated with various diseases was significantly lower than that of patients without kidney-yang deficiency syndrome [3]. In addition, clinical studies have reported that serum testosterone in elderly male patients with kidney-yang deficiency or kidney deficiency is relatively lower and serum estradiol is relatively higher. Further, the serum testosterone/ estradiol ratio is significantly lower than that of young males [20-22]. In the present study, the relative levels of serum testosterone and estradiol as well as the serum testosterone/estradiol ratio in ORC rats compared with those in the YRC group was similar to those shown in the clinical reports mentioned above. Judging from the interest index or activity ability, urinary 17-OHCS level, serum testosterone and estradiol levels and the serum testosterone/estradiol ratio, the rats in the ORC group were in the state of kidney-yang deficiency. After treatment using suspended moxibustion, all the previously mentioned values showed significant improvement compared with those seen before treatment.
In the present study we have found that, compared with those in the YRC group, serum levels of ACTH, CRH and CORT in the ORC group all decreased significantly, further indicating that the aged rats were in the state of KYDS. This seems to suggest that the HPA axis function in ORC rats decreased significantly compared with that of the YRC group. The results in this study are similar to our previous reports [13,23]. The primary GC in primates is cortisol which is corticosterone in rodents [24]. In the HPA axis feedback loop, GC feedback inhibits ACTH and CRH synthesis [25,26]. When KYDS occurs during aging, the overall function of the neuroendocrine/immune network in the elderly declines leading to a decrease in secretion of CRH synthesized by hypothalamic neurons which, in turn, affects secretion of ACTH and CORT [13,23].
Two kinds of receptor, Glucocorticoid (GRs) and Mineralorticoid Receptors (MRs), are abundant in the hippocampus. 11β-HSD 1 is highly expressed in the hippocampus, amygdala, neocortex and cerebellum of adult rats [27]. 11 Beta-Hydroxysteroid Dehydrogenase (11β-HSD) is a micro-enzyme complex which is the glucocorticoid rate-limiting enzyme. It catalyzes the REDOX reaction between glucocorticoid ketogroups (inactive) and hydroxyl groups (active) at carbon position 11 [28]. 11β-HSD has two isoenzymes, 11β-HSD 1 and 11β-HSD 2. 11β-HSD 1is a primary reductase that converts inactive 11-dehydrocorticosterone/cortisone into active CORT/cortisol. The action of 11β-HSD 2 is opposite to that of 11β-HSD 1 [29]. It affects GC activity by pre-receptor regulation and thus regulates the binding of MR, GR and GC in the hippocampus, and indirectly affects the normal function of the hippocampus. Carbenoxolone (CBX), a derivative of glycyrrhiza, is a non-selective inhibitor of 11β-HSD that can inhibit both 11β-HSD 1 and 11β-HSD 2. In this study, the expression of MR, GR and 11β-HSD 1 in the hippocampus of rats in the ORC group was all significantly decreased compared with that in the YRC group. After treatment with moxibustion, the expression of MR, GR and 11β-HSD 1 was significantly higher than that of the ORC group. Our results are similar to previous reports. Yao found that mRNA expression of MRs and GRs in the hippocampus of aged rats was significantly lower than that of young rats. After treatment with yougui pills or Shenqi pills, MRs and GRs mRNA expression was significantly increased compared with aged control rats [30,31].
According to traditional Chinese medicine, moxibustion functions to warm the body, warming the kidney and strengthening the yang. shenshu is mainly used to repair yang, guanyuan is mainly used to repair Qi, and the two acupoints are in harmony with each other so as to cultivate yuan qi and improve function by tonifying the kidney and warming the yang [32]. In this study, compared with those in the ORC group, the expression of MR, GR and 11β-HSD1 mRNA and protein in the hippocampus of rats were significantly up-regulated by suspended moxibustion treatment on the shenshu and guanyuan points. Serum levels of CRH, ACTH and CORT were significantly increased by suspended moxibustion treatment. One possible reason for this is that, according to TCM theory, the shenshu point belongs to the bladder meridian which is the back shu point of the kidney. The guanyuan point belongings to ren meridian, which is the small intestine collection point, and is the commonly used point for treatment of a variety of deficiencies. Another cause for this effect may be that, according to the modern medical theory, the shenshu point is closely related to the kidney, adrenal gland and internal genitalia through the somatic and sympathetic nerves. The guanyuan point is connected with the liver, spleen, kidney and other substantial organs through the twelve thoracic nerves and associated small visceral nerves. For example, Zhou using HRP nerve tract tracking technology, found that afferent projections of the guanyuan and uterus showed convergence and overlap in the spinal ganglia between lumbar 3 and sacral 5 [33]. In the present study, treatment with suspended moxibustion on shenshu and guanyuan not only has a strong theoretical basis, but also a wide range of experimental support. For example, when Zhao conducted mild moxibustion treatment on the guanyuan and shenshu points in elderly patients with KYDS, the symptoms of KYDS improved over time [34]. Ren showed that inhibition of the HPA axis in rats with KYDS was significantly improved after moxibustion treatment. The therapeutic effect of the shenshu and guanyuan combination was significantly better than that of the shenshu and zusanli or zusanli and guanyuan combination [35].
In summary, when moxa sticks are suspended at the shenshu and guanyuan points, the mild stimulation of heat from the burning moxa is transformed into electrical signals that are directly transmitted to the brain through afferent nerve pathways. After integration within the brain, it affects the hippocampus and other nerve nuclei and finally the HPA axis. Peripheral stimulation can also have a direct effect on visceral organs through visceral sensory nerves connected with the trafficking branches and finally affect the hippocampal-HPA axis through the sympathetic nerve chain. Finally, the use of moxibustion on the shenshu and guanyuan acupoints can promonte treatment of KYDS in aged rats by up-regulating expression of MR, GR and 11β-HSD 1 mRNA and protein in hippocampus, and increasing serum levels of CRH, ACTH and CORT. Because the kidney-yang deficiency syndrome in the aged rats used in the present study shares several pathologic similarities to that in humans, MR, GR and 11β-HSD 1 could represent novel landmarks for therapeutic targets in the diagnosis and treatment of frailty.
This work was supported by the National Natural Science Foundation of China (No. 81660817).
HHY conceived and designed the study and revised the paper. YJM wrote the paper, provided data and revised the paper. LW, LHC, ESH participated the experiment. All authors approved the final version of the paper.
None declared.
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Min Y, Yao H, Wang L, Cheng L, Hong E (2022) Effects of Suspended Moxibustion on the Expression of MR, GR and 11β-HSD1 in Hippocampus of Aged Rats. Endocrinol Metab Syndr.11:367.
Received: 06-Dec-2022, Manuscript No. EMS-22-20722; Editor assigned: 08-Dec-2022, Pre QC No. EMS-22-20722; Reviewed: 19-Dec-2022, QC No. EMS-22-20722; Revised: 30-Dec-2022, Manuscript No. EMS-22-20722; Published: 06-Dec-2022 , DOI: 10.35248/2161-1017-22.11.367
Copyright: © 2022 Min Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.