Endocrinology & Metabolic Syndrome
Open Access
ISSN: 2161-1017
ISSN: 2161-1017
Research Article - (2020)Volume 9, Issue 4
Regulatory roles of SSAs in catecholamine synthesis have not been elucidated. To clarify the actions of SSAs on catecholamine biosynthesis, we investigated the mutual interactions among SSAs including octreotide and pasireotide, steroids and BMPs using rat pheochromocytoma PC12 cells. Treatment with octreotide and pasireotide (10 nM to 10 μM) had no significant effect on mRNA levels of Th, DOPA decarboxylase and dopamine-β-hydroxylase in PC12 cells. Regarding the interaction with steroids, treatments with SSAs also had no effect on dexamethasone- or aldosterone-induced Th mRNA expression, while pasireotide reduced mRNA expression of the GR. As for the interaction with BMP-4, which can suppress Th mRNA expression by PC12 cells, SSAs did not affect Th expression reduced by BMP-4 and Id1 or Smad1/5/9 activation induced by BMP-4. However, BMP-4 treatment up-regulated MR expression, while treatment with noggin, which neutralizes endogenous BMPs, downregulated MR expression, and the presence of noggin also attenuated aldosterone-induced Th expression, suggesting that endogenous BMPs act to enhance MR activity. Moreover, BMP-4 treatment suppressed the expression of somatostatin receptors including Sstr2 and Sstr5 in PC12 cells, while treatment with noggin up-regulated the expression of Sstr2 and Sstr5, suggesting that BMPs play a desensitizing role in SSA actions. Collectively, the results revealed that SSAs have no direct effect on catecholamine synthesis; however, adrenomedullar BMPs could be modulators for the responsiveness to MR and SSTRs.
Bone morphogenetic proteins; Catecholamines; Corticosteroids; Somatostatin analogs
Aldo: Aldosterone, BMP: Bone Morphogenetic Protein, Dex: Dexamethasone, DHT: Dihydrotestosterone, DDC: DOPA Decarboxylase, DBH: Dopamine-β-hydroxylase, E2: Estradiol, GR: Glucocorticoid Receptor, MR: Mineralocorticoid Receptor, Oct: Octreotide, Pas: Pasireotide, PPGL: Pheochromocytoma/ paraganglioma, SSA: Somatostatin Analog, SSTR: Somatostatin Receptor, TH: Tyrosine Hydroxylase
Pheochrmocytoma/paraganglioma (PPGL) is neuroendocrine tumor that originates from chromaffin cells in the adrenal medulla. Clinicians and researchers now consider that PPGLs have metastatic potential and should be managed as malignant tumors [1]. One major problem for PPGLs is the management of various symptoms that arise from tumor-producing catecholamines. Although adrenergic antagonists can relieve many catecholamineinduced symptoms, their effects are often insufficient for patients with advanced PPGLs. Catecholamines, represented by dopamine, noradrenaline, and adrenaline, are synthesized from tyrosine in vivo. TH converts tyrosine to 3,4-dihydroxyphenylalanine, which is followed by conversion by DOPA decarboxylase of DOPA to dopamine, conversion by dopamine-β-hydroxylase of dopamine to noradrenaline, and conversion by phenylethanolamine N-methyltransferase of noradrenaline to adrenaline. In this cascadic process, the action of TH is considered to be a ratelimiting step [2]. Metyrosine, which is a direct inhibitor of TH, has been shown to reduce catecholamine secretion by PPGLs [3]. Nevertheless, some patients showed no response to or intolerance to metyrosine. Therefore, medication with higher efficacy and potency to suppress catecholamine production and secretion is required. SSAs, such as Oct, lanreotide, and Pas, are peptidic agents that suppress secretion of hormones and proliferation of neuroendocrine tumors. SSAs have been commonly used for treatment of neuroendocrine tumors and functional pituitary tumors including acromegaly and Cushing’s disease [4-7]. However, SSAs have not been practically applied for the control of functioning PPGLs, despite the fact that the expression of SSTRs on cell surfaces of PPGLs has been confirmed [8,9]. The effects of SSAs on PPGLs have been investigated only in small cohorts of patients, in which it was shown that SSAs were effective for suppressing catecholamine secretion from pheochromocytomas [10,11]. In contrast, it was also reported that SSAs could not reduce or even increased catecholamine secretion [12-14].The effectiveness of SSAs for reduction of catecholamine synthesis has yet to be established. Several agents have been shown to modulate catecholamine synthesis. For instance, cortisol and aldosterone are known as catecholamine-inducing hormones [15,16]. These corticosteroids are synthesized in the adrenal cortex and secreted into the bloodstream and are firstly delivered to adrenomedullar tissues. As a result, medullary cells are physiologically and locally exposed to corticosteroids at high concentrations, leading to induction of catecholamine synthesis [17]. We previously reported regulatory effects of BMPs, which were initially identified as bonedifferentiating factors and are currently thought to be involved in differentiation and endocrine regulation in various tissues, on catecholamine production [18]. In our previous studies, BMP-4 was found to be a modulator for catecholamine synthesis by rat pheochromocytoma cells [16,19,20]. However, the functional interaction for catecholamine biosynthesis between SSAs and BMPs has not been elucidated. The aims of the present study were to determine whether SSAs can suppress catecholamine synthesis and to clarify the underlying mechanisms interacting among the factors involved in catecholamine biosynthesis including SSAs, BMPs and corticosteroids. This is the first report that highlights the change of catecholamine synthesis caused by SSAs. The findings in this study expand our knowledge regarding the possibility of SSA application for control of catecholamine biosynthesis.
Reagents and supplies
Dulbecco’s Modified Eagle Medium with 0.45%(w/v) glucose, fetal bovine serum, penicillin/streptomycin solution, dexamethasone, aldosterone, estradiol, and dihydrotestosterone were purchased from Sigma-Aldrich Corp. (St. Louis, MO). Horse serum (HS) was obtained from Thermo Fisher Scientific Inc. (Waltham, MA). BMP-4, -7, and -9 were obtained from R&D Systems Inc. (Minneapolis). Octreotide and pasireotide were provided by Novartis International Pharmaceutical Ltd. (Basel, Switzerland). Anti-phospho-Smad1/5/9 (pSmad1/5/9) antibody, anti-Smad1 (tSmad1) antibody,and horseradish peroxidase-linked anti-rabbit IgG antibody were purchased from Cell Signalling Technology, Inc. (Beverly, MA). Anti-actin antibody was obtained from Sigma- Aldrich Corp.
Cell culture procedure
PC12 cells were obtained from RIKEN (Saitama, Japan). The catalog number of the cells was RCB0009. The cells of passages 5 to 15 were cultured in DMEM supplemented with 10%(v/v) FBS, 10%(v/v) HS, and 1%(v/v) PS at 37°C in 5%(v/v) of CO2. Regarding FBS and HS, we conducted heat inactivation according to the method recommended by the provider. Cell cultures were passaged when confluences reached about 80%.
Reverse transcription and quantitative polymerase chain reaction (RT-qPCR)
Five hundred thousand viable PC12 cells were treated with the indicated reagents in DMEM containing 1 %(v/v) FBS, 1%(v/v) HS, and 1%(v/v) PS. After incubation for 24 h to 48 h, total RNA was extracted using TRI reagent® (Molecular Research Center Inc., Cincinnati, OH). The RNA was purified by isopropanol precipitation and its yields were measured by a NanoDrop™ One spectrophotometer (Thermo Fisher Scientific Inc.). One μg of purified total RNA was subjected to 9 reverse transcriptions with ReverTra Ace® (Toyobo, Co., Ltd., Osaka, Japan). Primer pairs for Th, Ddc, Dbh, Gr, Mr, Id1, Sstr2, Sstr5 and Rpl19 were selected from different exons of corresponding genes to avoid amplifying genomic DNA [16,19,21]. After optimizing the annealing conditions for each pair of primers, qPCR was performed to quantify the level of target gene mRNA using the LightCycler® Nano real-time PCR system with SYBR® Green dyes (Roche Diagnostics GmbH, Basel, Switzerland). The relative expression of each mRNA was determined by the ΔCt method, in which ΔCt is the value obtained by subtracting the Ct value of ribosomal protein L19 (Rpl19) mRNA from that of the target mRNA. The amount of target mRNA relative to Rpl19 mRNA was expressed as 2-(ΔCt), and the results were expressed as the ratio of target mRNA to Rpl19 mRNA.
Western blots for Smads
One hundred thousand viable PC12 cells were pretreated with 1 μM of Oct or Pas in DMEM containing no serum and 1%(v/v) PS. After 24-h incubation, the cells were stimulated with BMP-4 (30 ng/ml) for one hour and then they were lysed in 100 μl of buffer containing 0.05 M tris (hydroxymethyl) aminomethane hydrochloride (pH 7.4), 0.15 M sodium chloride, 0.25%(w/v) deoxycholic acid, 1%(v/v) NP-40, 1 mM ethylenediaminetetraacetic acid, and 0.05%(v/v) β-mercaptoethanol. The samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis using Invitrogen™ NuPAGE™ 4-12% Bis-Tris Gel (Thermo Fisher Scientific Inc.) and then proteins were electroblotted onto polyvinylidene difluoride membranes. The membranes were blocked by 5%(w/v) bovine serum albumin and incubated with primary antibodies (anti-pSmad1/5/9, tSmad1 and actin antibodies) and then with secondary antibodies. Can Get Signal® Immunoreaction Enhancer Solution (Toyobo, Co., Ltd.) was also used to accelerate the antigen-antibody reaction. The integrated band intensities were measured by the C-DiGit® Blot Scanner System (LI-COR Biosciences, Lincoln, NE) and the data of pSmad1/5/9 were normalized by the data of tSmad1.
Statistical analysis
All results are shown as means ± standard errors of the mean (SEM) of data from at least three independent experiments with triplicated samples. The data were subjected to statistical calculation for analysis of variance (ANOVA) with Tukey-Kramer’s post hoc test and unpaired t-test performed by using GNU R version 3.3.1 (R Development Core Team). A p value < 0.05 was shown to be statistically significant.
First, we examined the effects of SSAs on the expression levels of catecholamine-producing enzymes in PC12 cells. As shown in Figure 1, treatments with a wide range of concentrations (10 nM to 10 μM) of Oct or Pas had no significant effect on catecholamineproducing enzyme mRNA levels including the genes of Th, Ddc, and Dbh in PC12 cells. Pnmt mRNA levels were not assessed in this study because of the lack of Pnmt expression in PC12 cells [22]. Next, we examined the effects of SSAs on catecholamine biosynthesis by PC12 cells induced by various steroids (0 to 100 nM). As shown in Figure 2A, treatment with Dex (10-100 nM), Aldo (100 nM) and E2 (10 nM), but not treatment with DHT, significantly increased Th mRNA expression by PC12 cells. The increases in Th mRNA expression levels were highest by treatments with Dex (100nM) and Aldo (100 nM) with increases by 5 fold and 1.5 fold, respectively, among the four steroids examined in this experiment. We therefore selected Dex and Aldo to investigate the interactions between SSAs and steroids for catecholamine synthesis. It was found that the mRNA expression levels of GR and MR were moderately reduced by treatments with Oct and Pas (1 μM), but the suppressive effect of Pas on GR expression was statistically significant (Figure 2B). However, treatment with Pas and Oct did not affect the level of Th mRNA expression induced by Dex (10 nM) or Aldo (100 nM) (Figure 2C). We next assessed the interactions between SSA activity and BMPs for catecholamine biosynthesis by PC12 cells. As shown in Figure 3A, among the BMP ligands including BMP-4, -7, and -9, treatment with BMP-4 (30 ng/ml) elicited a significant reduction of Th mRNA expression. Treatment with Oct and Pas (1 μM) did not affect levels of Th mRNA expression suppressed by BMP-4 (30 ng/ml) and also it did not change the BMP-4-induced transcription of Id1 mRNA. In addition, the phosphorylation of Smad1/5/9 induced by BMP-4 (30 ng/ml) was not altered by treatment with Oct or Pas (1 μM). We further approached the interactions between corticosteroids and BMPs for regulating catecholamine synthesis by PC12 cells. As shown in Figure 3B & 3C, it was revealed that treatment with BMPs (30 ng/ml) modulated mRNA expression of GR and MR: BMP-7 (30 ng/ml) down regulated GR expression (Figure 3B) while BMP-4 and BMP-9 (30 ng/ml) upregulated MR expression (Figure 3C) by PC12 cells. Treatment with noggin (30 ng/ml), a BMP-binding protein, suppressed mRNA expression of GR (Figure 3B) and MR (Figure 3C) for 24- to 48-h culture, suggesting that endogenous BMPs seem to act to up regulate GR and MR. Given that treatment with noggin (30 ng/ml) abolished Aldo (100 nM)-induced (Figure 3D & 3E), but not Dex (10nM)- induced, Th mRNA expression (Figure 3D), endogenous BMPs are functionally involved in the up regulation of MR in PC12 cells. Finally, we investigated the interactions between SSAs and BMPs in PC12cells. As shown in Figure 4A, the expression levels of Sstr2 and Sstr5, which are key receptors for Oct and Pas, were not significantly altered by either treatment with Dex (10 nM) or Aldo (100 nM).Of note, the mRNA levels of Sstr2 and Sstr5 were significantly decreased by treatment with BMPs (Figure 4B). Namely, treatments with BMP-4, -7 and -9 (30 ng/ml) all reduced Sstr2 expression, while treatment with BMP-4, but not BMP-7 or -9, suppressed Sstr5 expression in PC12 cells. On the other hand, noggin treatment (30 ng/ml) increased mRNA levels of Sstr2 and Sstr5 (Figure 4B), suggesting that endogenous BMPs act to down regulate the expression of Sstr2 and Sstr5 in PC12 cells.
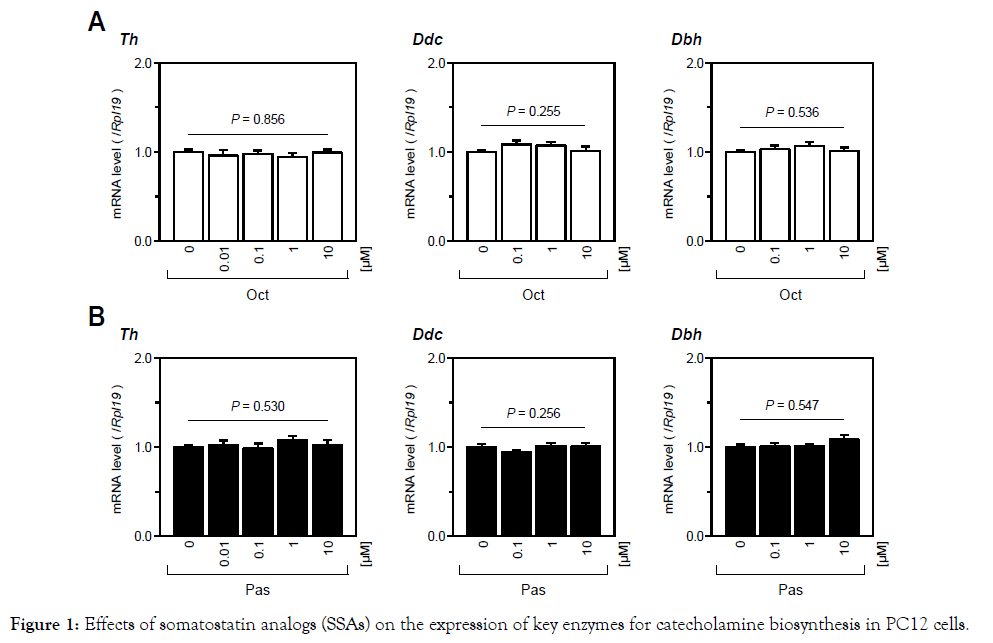
Figure 1. Effects of somatostatin analogs (SSAs) on the expression of key enzymes for catecholamine biosynthesis in PC12 cells.
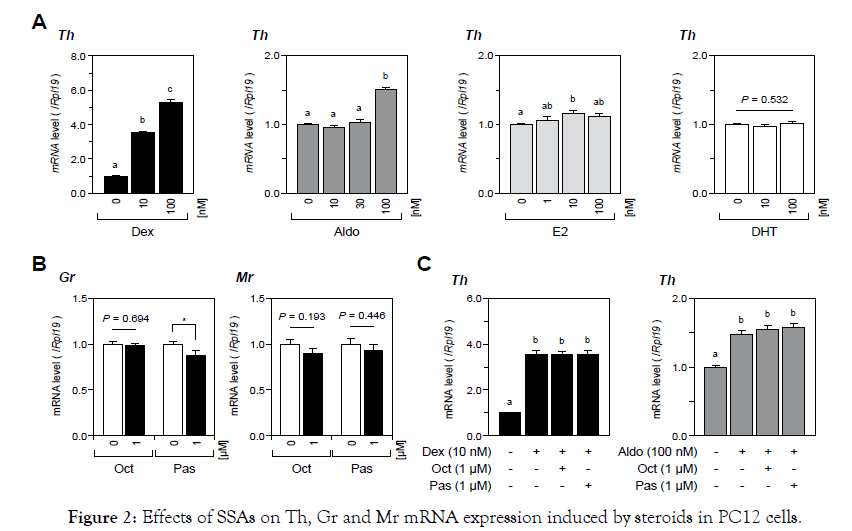
Figure 2. Effects of SSAs on Th, Gr and Mr mRNA expression induced by steroids in PC12 cells.
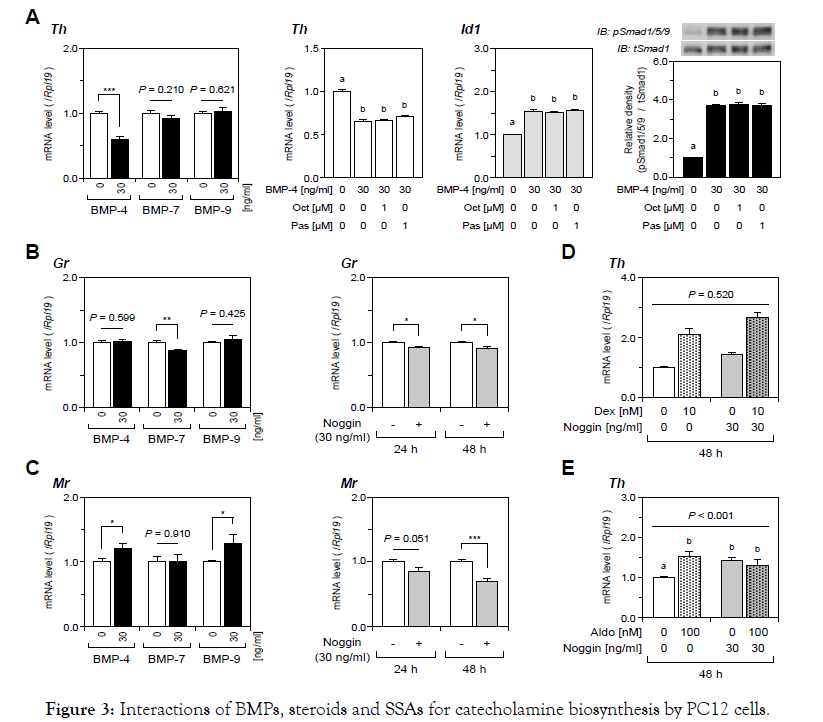
Figure 3. Interactions of BMPs, steroids and SSAs for catecholamine biosynthesis by PC12 cells.
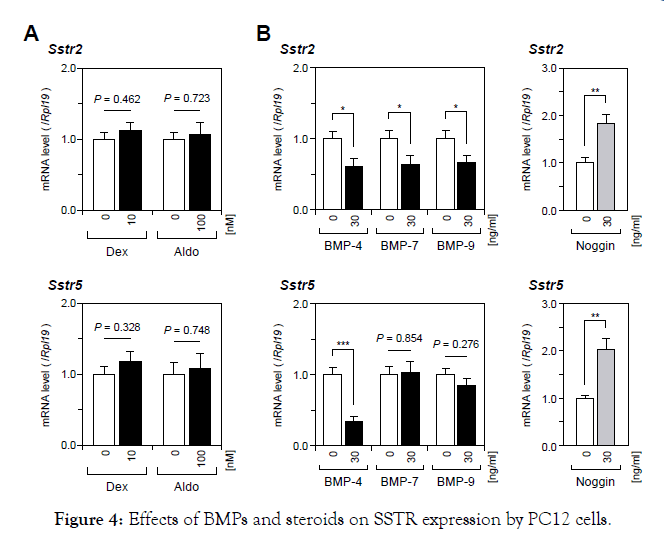
Figure 4. Effects of BMPs and steroids on SSTR expression by PC12 cells.
PC12 cells were cultured in DMEM containing 1%(v/v) FBS, 1%(v/v) HS, 1%(v/v) PS, and indicated concentrations of SSAs including (A) Oct and (B) Pas for 24 h. Subsequently, total RNA was extracted and subjected to RT-qPCR analysis to determine the gene expression levels for Th, Ddc and Dbh mRNA. The expression levels of target genes were standardized by the Rpl19 level in each sample. All results are shown as means ± SEM of data from at least three independent experiments with triplicated samples. The results were analyzed by one-way ANOVA.
PC12 cells were cultured with DMEM containing 1%(v/v) FBS, 1%(v/v) HS, 1%(v/v) PS, and indicated concentrations of (A) steroids, (B) SSAs, and (C) steroids and SSAs for 24 h. Subsequently, total RNA was extracted and subjected to RT-qPCR analysis to determine the gene expression levels of Th, GR and MR mRNA. The expression levels of target genes were standardized by the Rpl19 level in each sample. All results are shown as means ± SEM of data from at least three independent experiments with triplicated samples. The results were analyzed by one-way ANOVA or the unpaired t-test. Different superscript letters mean a significant difference at P<0.05. Asterisks mean a significant difference from the control group: *P<0.05.
PC12 cells were cultured in DMEM containing 1%(v/v) FBS, 1%(v/v) HS, 1%(v/v) PS, and indicated concentrations of (A) BMPs and SSAs and (B, C) BMPs and noggin for 24 h to 48 h. D, E) Cells were treated with noggin for 24 h and then stimulated with indicated concentrations of steroids for another 24 h. Subsequently, total RNA was extracted and subjected to RT-qPCR analysis to determine the gene expression of Th, Id1, Gr and Mr mRNA. The expression levels of target genes were standardized by the Rpl19 level in each sample. For protein analysis (A), cells were cultured in DMEM containing 1%(v/v) PS with SSAs for 24 h and then stimulated with BMP-4 for 1 h, and the cell lysates were analyzed by Western blotting to evaluate phosphorylation of Smad1/5/9. All results are shown as means ± SEM of data from at least three independent experiments with triplicated samples. The results were analyzed by the unpaired t-test (A-C), one-way ANOVA (A), or two-way ANOVA (D, E). Different superscripts denote a significant difference at P<0.05. Asterisks mean a significant difference from the control group: *P<0.05, **P<0.01 and ***P<0.001.
PC12 cells were cultured in DMEM containing 1%(v/v) FBS, 1%(v/v) HS, 1%(v/v) PS, and indicated concentrations of (A) steroids and (B) BMPs and noggin for 24 h. Subsequently, total RNA was extracted and subjected to RT-qPCR analysis to determine the gene expression levels of Sstr2 and Sstr5 mRNA. The expression levels of target genes were standardized by the Rpl19 level in each sample. All results are shown as means ± SEM of data from at least three independent experiments with triplicated samples. The results were analyzed by the unpaired t-test. Asterisks mean a significant difference from the control group: *P<0.05; **P<0.01; and ***P<0.001.
One of the main goals of the present study was to reveal the effects of SSAs on catecholamine biosynthesis in vitro. The results of the present study showed that neither Oct nor Pas, at concentrations from 10 nM to 10 μM, altered mRNA expression levels of key enzymes for catecholamine biosynthesis including Th, Ddc and Dbh. Th is thought to be a rate-limiting enzyme for catecholamine biosynthesis, and the net activity of Th is regulated by its gene transcription and phosphorylation of Th [23]. It has been shown that there is a strong correlation between the amount of secreted catecholamines and mRNA levels of the Th gene [24]. Therefore, we evaluated the capability for catecholamine biosynthesis based on the expression levels of Th mRNA in this study. Since no significant change of Th expression was detected under the condition of treatment with SSAs in the presence or absence of corticosteroid stimulation, SSA was not considered to be a direct modulator for catecholamine biosynthesis in vitro. The possibility of whether SSAs can reduce only the secretory process without suppression of the synthetic process of catecholamines is an important point of discussion. In this regard, Invitti et al. [10] reported a transient reducing effect of Oct infusion for 2 hours on plasma noradrenaline levels in patients with PPGLs. Pasquali et al. [11] showed suppressive effects of SSAs on primary cells derived from PPGL tissues in vitro, in which Oct and, in particular, Pas were effective for induction of cellular apoptosis and reduction of cellular catecholamine contents. On the other hand, Lamarre-Cliche et al. [14] reported that chronic therapy with slowreleasing Oct for 3 months failed to reduce plasma catecholamine levels in patients with malignant or recurrent PPGLs.It is thus possible that SSAs can temporally suppress the secretory process of catecholamines but that SSAs cannot have long-lasting effects since the results of the present study showed that SSAs had no effect on the upstream process regulating Th expression for catecholamine production. Another goal of the present study was to elucidate the interactions among SSAs, BMPs and corticosteroids in terms of catecholamine biosynthesis. Regarding the interaction of catecholamines and corticosteroids, endogenous glucocorticoids are known to induce catecholamine biosynthesis by stimulating catecholamine-synthesizing enzymes though the cortico-medullary portal system [25]. Increased expression levels of Pnmt [25] and Th [26] and augmented catecholamine release are also involved in this mechanism. As for the interrelationships between SSAs and corticosteroids, the present study demonstrated that treatment with Pas, but not treatment with Oct, moderately reduced GR expression on PC12 cells. However, neither of the SSAs significantly reduced Th expression induced by corticosteroids. This might be due to weak suppression of GR and MR expression by SSAs. The differential affinity of SSAs to SSTRs might also be involved in this result. Namely, SSAs act through five subtypes of SSTRs including subtype-1 to subtype-5, and Oct has a high affinity to SSTR-2, while Pas has a relatively high affinity to SSTR- 2 and -5 [27]. This difference might be linked to the differential actions of SSAs on the modulation of GR/MR expression in PC12 cells. Given that glucocorticoids are thought to induce adrenaline synthesis by upregulating Pnmt expression in the human adrenal medulla, our results indicate that Pas is a possible agent for attenuating catecholamine synthesis by downregulating GR [28]. As for the interactions between SSAs and BMPs for catecholamine biosynthesis, we found that Sstr2 and Sstr5 were downregulated by BMP treatments. BMP-4 suppressed mRNA expression of Sstr2 and Sstr5, while BMP-7 and -9 suppressed the expression of only Sstr2. Since it has been shown that BMP-4 and -7 are expressed in PC12 cells, these BMP ligands were considered to be endogenous BMPs in PC12 cells [19,29]. In fact, treatment with noggin, a binding protein for BMPs, resulted in induction of Sstr2 and Sstr5 expression in PC12 cells. These results indicated that endogenous BMPs including BMP-4 and -7 down regulated SSTRs, leading to impaired activity of SSAs. Accordingly, the expression levels of endogenous BMPs might be critical for the effectiveness of SSAs. Regarding the interactions between SSAs and BMPs, we previously reported the effects of SSAs on ovarian steroidogenesis by rat granulosa cells [30]. SSTR activation was found to modulate steroidogenesis by up regulating endogenous BMP activity via suppression of inhibitory Smads6/7 expression in ovarian cells. In cases of interaction with pituitary BMPs, treatments with BMP- 4 and -6 regulated the expression levels of SSTR-2 and -5 in rat GH3 cells and mouse LβT2 cells, suggesting a role of BMPs in modulation of SSA sensitivities [31,32]. A comparison of these findings with results of the present study in adrenomedular cells suggests that functional interaction between SSAs and the BMP system is highly diverged in a tissue-specific manner. We also approached the interrelationship between activity of BMPs and corticosteroids in adrenomedullary cells. In the present study, BMP-4 and -9 increased Mr expression in PC12 cells, whereas noggin treatment suppressed MR expression and the following Th induction elicited by Aldo, suggesting that endogenous BMP- 4 is likely to be an enhancer for mineralocorticoid action via up regulation of MR. Unlike BMP-4 which acts as a local factor, BMP- 9 is mainly produced in the liver and is systemically delivered, and BMP-9 may therefore continuously affect catecholamine synthesis in vivo [33,34]. Of interest, among the BMP ligands we used, only BMP-7 reduced GR expression just like Pas. Given that BMP-7 did not suppress the expression of Sstr5, which is a key receptor for Pas, a combined effect of Pas with enhanced BMP-7 could be an effective candidate for controlling catecholamine biosynthesis induced by glucocorticoids. However, a main limitation of the present study is that the effects of SSAs on cell proliferation were not evaluated. A previous study showed that SSAs suppressed the growth of neuroendocrine tumors [5]. In fact, octreotide has been used as an anti-cancer agent for treatment of a neuroendocrine tumor. Since the total amount of catecholamine and the volume of PPGL are generally correlated, controlling tumor growth could lead to regulation of catecholamine production. In conclusion, we revealed that major SSAs did not have direct effects on gene expression of catecholamine-synthesizing enzymes in PC12 cells. Given that BMPs modulate the expression of SSTRs and steroid receptors, the existence of endogenous BMPs are important for tuning of SSA sensitivity. The SSTR actions for regulating catecholamine synthesis seem to be indirect, but endogenous BMP activity could be a determinant for the sensitivity to SSAs and steroids in the adrenal medulla.
This work was supported in part by Grants-in-Aid for Scientific Research (No. 18K08479), Ofuji Endocrine Medical Award (Japan), Ryobi Teien Memory Foundation Award (Japan), Forum on Growth Hormone Research Award (Japan), and Japan Foundation for Applied Enzymology.
The author(s) declared no conflicts of interest with respect to the authorship or the publication of this article.
There is nothing to disclose.
Citation: Nada T, Komatsubara M, Iwata N, Nakano Y, Otsuka F (2020) Effects of Somatstatin Analogs on catecholamine Biosynthesis Regulated by Corticosteroids and Bone Morphogenetic Proteins in Rat Pheochromocytoma cells. Endocrinol Metab Syndr 2020; 9:307. doi: 10.35248/2161-1017.20.9.307
Received: 01-Jul-2020 Accepted: 15-Jul-2020 Published: 22-Jul-2020 , DOI: 10.35248/2161-1017.20.9.307
Copyright: ©2020 Nada T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This work was supported in part by Grants-in-Aid for Scientific Research (No. 18K08479), Ofuji Endocrine Medical Award (Japan), Ryobi Teien Memory Foundation Award (Japan), Forum on Growth Hormone Research Award (Japan), and Japan Foundation for Applied Enzymology.