Reproductive System & Sexual Disorders: Current Research
Open Access
ISSN: 2161-038X
ISSN: 2161-038X
Research Article - (2021)Volume 10, Issue 4
Background: Lead and its compounds have been implicated to be reprotoxic by inducing testicular damage and causing hormonal imbalance. Senna podocarpa has antimicrobial, anti-inflammatory and wound healing potentials. This study investigated the effects of the methanol extract of Senna podocarpa leaf (MESP) on the reproductive activities of male Wistar rats following the induction of reproductive toxicity using lead acetate.
Methods: Thirty male rats with body weights between 170g - 200g (10 - 12 weeks) were used for the study. The rats were divided into 6 equal groups as follows; Group 1 rats received 1 ml/kg of Tween 80 only while group 2 rats received 15 mg/kg lead acetate only. Rats in group 3 received 15 mg/kg lead acetate and 500mg/kg of MESP concurrently while rats in group 4 received 15 mg/kg lead acetate and 1000mg/kg of MESP concurrently. Groups 5 and 6 rats received only 500 mg/kg and1000 mg/kg of MESP respectively. Lead acetate was administered intraperitoneally while MESP and Tween 80 were administered orally. All treatments were for 14 days.
Results: It was observed from the results that administration of lead acetate for 14 days induced male reproductive toxicity which was evidenced by testicular and epididymal degeneration, significant decrease in sperm parameters, reproductive hormone and antioxidant level. However, concomitant administration of MESP especially at 1000 mg/kg ameliorated the toxic effect of lead acetate as shown by improved testicular and epididymal histology as well as increased sperm parameters.
Conclusion: Our findings suggest that MESP may be used as herbal remedy in cases of reproductive toxicity caused by lead acetate.
Senna podocarpa; Rat; Sperm; Testosterone; Lead acetate; Epididymis
The various deleterious effects to health as a result of exposure to heavy metals found in the environment pose a serious challenge and also a great global concern. Lead has been shown to be the most abundant toxic metal in the environment [1]. It does not have any beneficial biological role but its detrimental effect on physiological, biochemical and behavioral functions in animals and humans have been reported. [2-4]. It is an industrial and environmental pollutant that has been detected in every environmental and biological system. Lead is considered as one of the hazardous pollutants and toxins that are found in the environment. Most of the lead pollution in our environment comes from gasoline used in cars, industrial emission, drinking water, petroleum refining, in construction, lead batteries and has led to it contaminating the air, dust and soil. Human exposure to lead continues to be a serious public health problem [5]. Lead toxicity in the male reproductive system is manifested by the deposition of lead in the testes, epididymis, vas deferens and seminal vesicles [5,6]. It has been reported to disrupt the hypothalamic-pituitary-gonadal axis by reducing plasma Luteinizing hormone and testosterone level. These studies have shown that lead causes abnormalities of spermatogenesis which was evident by seminiferous tubule degeneration [7,8]. It also has Enzymeadverse effects on sperm count, sperm motility and sperm viability [9]. The use of herbs in the treatment of diseases dates back to ages [10,11].
However, traditional medicine, which has been in existence for quite a long time, was once believed to be primitive and met with great hostility. This hostility was shown by foreign religions, dating back to the colonial days in Africa and more recently by the conventional or orthodox medical practitioners [12]. More recently, traditional medicine has become very important in meeting some of the needs of primary health care delivery in Africa and in most countries of the world.
Senna podocarpa (Guill. & Perr) LOCK is a very popular plant in Nigeria formerly called Cassia podocarpa Guill. Et Perr. (Leguminosae-Caesalpinioideae). It is a glabrous shrub up to 5m high. It is widely distributed in West Africa especially in the savannah forest area of the region. The plant is found locally on old farmlands in Western and Northern regions of Nigeria. It has been extensively used as a purgative, labour inducer, anti-gonorrhea, guinea worm expellant, emmenagogue and ecbolic in the folklore medicine [13]. It also has antimicrobial, anti-inflammatory and wound healing potential [14]. The indiscriminate exposure of humans and animals to toxic substances is a menace in the everincreasing industrial economies especially in developing and underdeveloped countries. Some of the toxic substances are inform of metals and majority of them are classified as heavy metals. Lead and its compounds have been implicated for centuries to be both neurotoxic and reprotoxic. Lead acetate, a potent lead compound has also been reported to induce testicular damage and cause hormonal imbalance [4,15]. This study aimed at investigating the possible ameliorative actions of Senna podocarpa in male Wistar rats following induced reproductive toxicity using lead acetate.
Animal Use and Care
Thirty male albino Wistar rats weighing 170g - 200g (10 - 12 weeks) were obtained from the Animal Holding Unit, College of Health Sciences, Obafemi Awolowo University, Ile-Ife. They were kept in conventional ventilated plastic rat cages under standard laboratory conditions of 12 hour light/dark cycle. Rat feed (Ace Feeds, Osogbo) and water was provided ad libitum. The animals were cared for and the study was conducted complying with the “Guide for the Care and Use of Laboratory Animals” [16]. The dosage of lead acetate (BDH chemicals Ltd. Poole England (Prod 10142) (5927240C5009) used to induce reproductive toxicity in the present study was a modified regimen as described [17,18].
Experimental Design
After the acclimatization period, the rats were divided equally into six groups of five rats each. Group I rats received 1 ml/kg of Tween 80 orally/gavage (control). Rats in group II were administered 15 mg/kg BW lead acetate intraperitoneally [19,20]. Groups III and IV rats received 15 mg/kg BW lead acetate intraperitoneally together with 500 and 1000 mg/kg BW of methanol extract of Senna podocarpa leaf (MESP) orally respectively [21]. Rats in groups V and VI 500 and 1000 mg/kg BW of MESP orally respectively. All treatments were given for fourteen days [22] (Table 1) and the body weights of the animals were also assessed.
| Groups | Number of rats | Drug Administration | Number of days |
|---|---|---|---|
| I | 5 | 1 ml/kg Tween 80 (Vehicle) | 14 |
| II | 5 | 15 mg/kg BW lead acetate (alone) | 14 |
| III | 5 | 15 mg/kg BW lead acetate + MESP (500 mg/kg BW) | 14 |
| IV | 5 | 15 mg/kg BW lead acetate + MESP (1000 mg/kg BW) | 14 |
| V | 5 | MESP (500 mg/kg BW) (alone) | 14 |
| VI | 5 | MESP (1000 mg/kg BW) (alone) | 14 |
Table 1: Dose regimen table.
Animal Sacrifice
Twenty-four hours after the last drug administration, the body weight of rats from each group was taken and they were sacrificed by cervical dislocation. The testes and epididymis were carefully removed and trimmed of the surrounding fats and also weighed using digital weighing balance (Camry balance, China). Their relative weights were then calculated.
Sperm Analysis
Sperm analysis was carried out as described by W.H.O. Briefly, progressive sperm motility and viability were done immediately after cervical dislocation. Progressive sperm motility was carried out by puncturing the caudal epididymis to release two drops of the semen on a pre-warmed microscope slide. Thereafter, two drops of citrate buffer solution (pH 7.4) were added and covered with a coverslip and examined under the microscope with low magnification (x40) and assessed. Sperm viability was carried out using the eosin/nigrosine stain. Two drops of semen were put on a microscope slide and two drops of the stain were added to it, covered with a coverslip, air-dried and observed using a light microscope. The dead sperms will be stained while the live sperms will not take up the stain. These will be counted and expressed as percentages [23]. Sperm morphology was carried out by placing two drops of semen on a microscopic slide and adding Walls and Ewas stain and air dried. The slide was then examined under a microscope with x100 magnification. A total of 100 sperms were evaluated and the abnormal sperms were expressed as a percentage. Sperm count was evaluated by mincing the caudal epididymis in a petri dish containing 2 ml of distilled water. The suspension was further diluted with sodium bicarbonate formalin solution at a ratio of 1:20. The new improved Neubauer’s hemocytometer counting chamber was filled with the diluted sperm solution, observed under the microscope and the number of sperm cells in 2 mm2 were counted, calculated and expressed in million/ml [15,24,25].
Hormonal and Antioxidant Assay
Blood was collected by cardiac puncture, transferred into plain sample bottles and separated by centrifuging in a cold centrifuge at 3000 rpm for 10 minutes at 4ºC (R-Series Cold Centrifuge, Centurion Scientific Ltd., West Sussek, U.K.) to obtain the serum. The serum luteinizing hormone (LH), follicle stimulating hormone (FSH) and testosterone were quantified using Enzymeadverse linked Immunosorbent Assay (ELISA) method following the manufacturer’s instructions (Inteco diagnostics reagents, UK). It was read using a microplate reader (Shanghai Yongchuang medical instrument Co., Model SM600, China) at a wavelength of 450 nm. Reduced glutathione (GSH) was measured using the method of Beutler et al. [26]. This was read using a microplate reader (Shanghai Yongchuang Medical Instrument Co., Model SM600, China) at a wavelength of 412 nm.
Histological Analysis
The left testes and epididymis were carefully excised for the sake of consistency and prefixed in Bouin-Hollande’s fluid to prevent putrefaction and autolysis. They were further processed and the sections (5 microns) were then stained with hematoxylin and eosin and examined using a light microscope [27].
Statistical Analysis
Data obtained were presented as mean ± standard error of mean (X ± SEM). Data normality was assessed by Kolmogrov-Smirnov test and the statistical significance between the control group and the treated groups was calculated by the analysis of variance (ANOVA) and post hoc analysis was carried out using Student-Newman-Keuls multiple comparison test using the Graph Pad 5.03 statistical software package (Graph Pad Software Inc., CA, USA). A probability value of p less than 0.05 was considered to be significant [28].
Body and Relative Organ Weights
A significant (p < 0.001) percentage weight loss was observed in rats in group II when compared to the control. Although, MESP alone at 500 and 1000 mg/kg had no significant effect on percentage weight gain (groups V and VI), both doses of MESP significantly reversed the lead induced reduction in the body weight of rats in groups III and IV. There was no significant change (p > 0.05) in relative testicular weight of rats across all the groups. There was a significant decrease (p < 0.01) in the relative epididymal weight of rats in group II compared with group I. However, MESP significantly increased the relative epididymal weight of rats in groups III and IV when compared to group II. There was no significant change in the relative epididymal weight of rats in groups V and VI when compared with the control (Table 2).
| Groups | Percentage weight change (%) | Testis (%) | Epididymis (%) |
|---|---|---|---|
| Group I [Control] | 9.33 ± 4.44 | 0.64 ± 0.03 | 0.38 ± 0.03 |
| Group II [15 mg/kg Lead acetate (LA)] | -6.21 ± 0.92α | 0.54 ± 0.05 | 0.21 ± 0.02 α |
| Group III [15 mg/kg LA+500 mg/kg MESP] | 5.73 ± 2.33β | 0.68 ± 0.03 | 0.41 ± 0.05 β |
| Group IV[15 mg/kg LA+1000mg/kg MESP] | 5.24 ± 1.05β | 0.62 ± 0.02 | 0.38 ± 0.03 β |
| Group V [500 mg/kg MESP] | 14.23 ± 3.62β | 0.55 ± 0.03 | 0.31 ± 0.02 |
| Group VI [1000 mg/kg MESP] | 11.67 ± 2.04β | 0.55 ± 0.05 | 0.35 ± 0.06 β |
Table 2: Effects of MESP on percentage weight change and relative weight of testis and epididymis of male Wistar rats following exposure to lead acetate.
Sperm Parameters
There was a significant decrease (p < 0.001) in progressive sperm motility of rats in group II, III, and IV when compared with the control. However, administration of MESP alone at 500 and 1000 mg/kg of the extract (groups V and VI) significantly increased their sperm motility when compared with group II (Figure 1). There was a significant decrease (p < 0.001) in sperm viability and count of rats in group II when compared with other groups. However, administration of MESP to rats in groups III, IV, V and VI significantly increased (p < 0.05) their sperm viability and count when compared to rats in group II (Figures 2 and 3). Administration of lead acetate significantly increased (p < 0.001) the number of abnormal sperms in groups II and III when compared with the control. However, treatment with MESP significantly decreased (p < 0.05) the number of abnormal sperms in groups III, IV, V and VI when compared with the rats in group II (Figure 4).
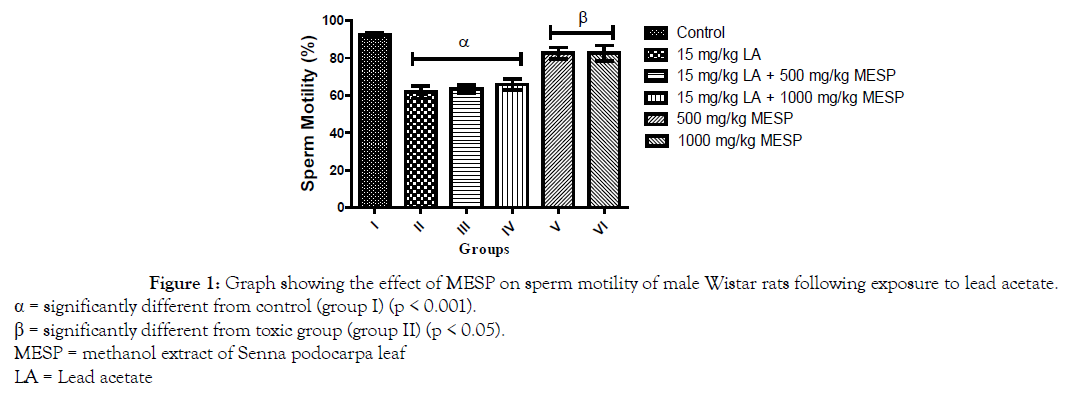
Figure 1. Graph showing the effect of MESP on sperm motility of male Wistar rats following exposure to lead acetate.
α = significantly different from control (group I) (p < 0.001).
β = significantly different from toxic group (group II) (p < 0.05).
MESP = methanol extract of Senna podocarpa leaf
LA = Lead acetate
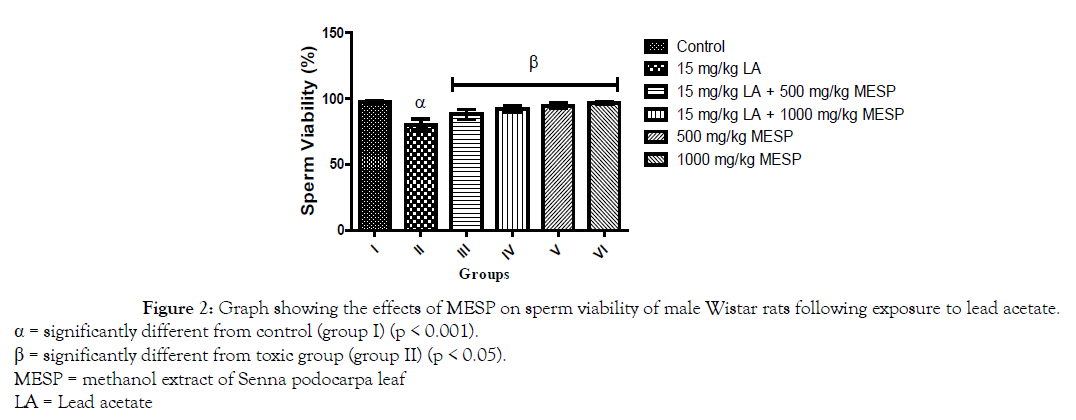
Figure 2. Graph showing the effects of MESP on sperm viability of male Wistar rats following exposure to lead acetate.
α = significantly different from control (group I) (p < 0.001).
β = significantly different from toxic group (group II) (p < 0.05).
MESP = methanol extract of Senna podocarpa leaf
LA = Lead acetate
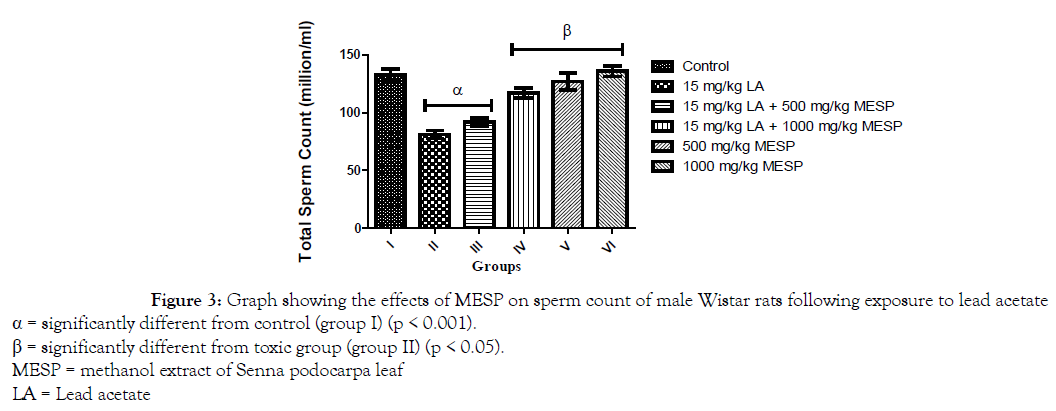
Figure 3. Graph showing the effects of MESP on sperm count of male Wistar rats following exposure to lead acetate
α = significantly different from control (group I) (p < 0.001).
β = significantly different from toxic group (group II) (p < 0.05).
MESP = methanol extract of Senna podocarpa leaf
LA = Lead acetate
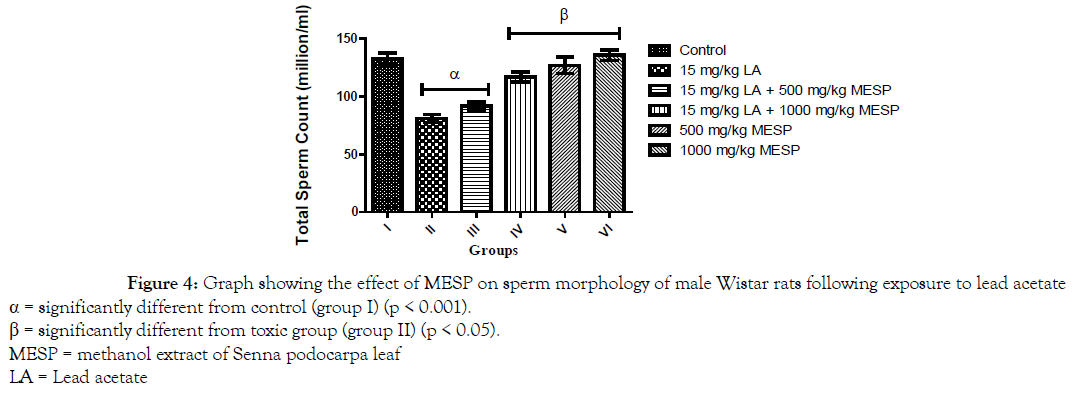
Figure 4. Graph showing the effect of MESP on sperm morphology of male Wistar rats following exposure to lead acetate
α = significantly different from control (group I) (p < 0.001).
β = significantly different from toxic group (group II) (p < 0.05).
MESP = methanol extract of Senna podocarpa leaf
LA = Lead acetate
Serum Hormonal and Antioxidant Concentrations
There was a significant decrease (p < 0.001) in the serum concentration of luteinizing hormone (LH) of rats in groups II and III when compared with the control group. However, a significant increase in serum LH was observed in groups IV, V and VI when compared with group II (Figure 5). Administration of lead acetate only to rats in group II significantly decreased (p < 0.001) their serum follicle stimulating hormone (FSH) concentration when compared with the control group. However, there was a significant increase in the serum FSH concentration of rats in groups III, IV, V and VI when compared to group II (Figure 6). A similar trend was observed in the serum testosterone and glutathione concentration of the rats. However, the glutathione concentration of rats in groups III and IV were significantly lower when compared to group I (Figures 7 and 8).
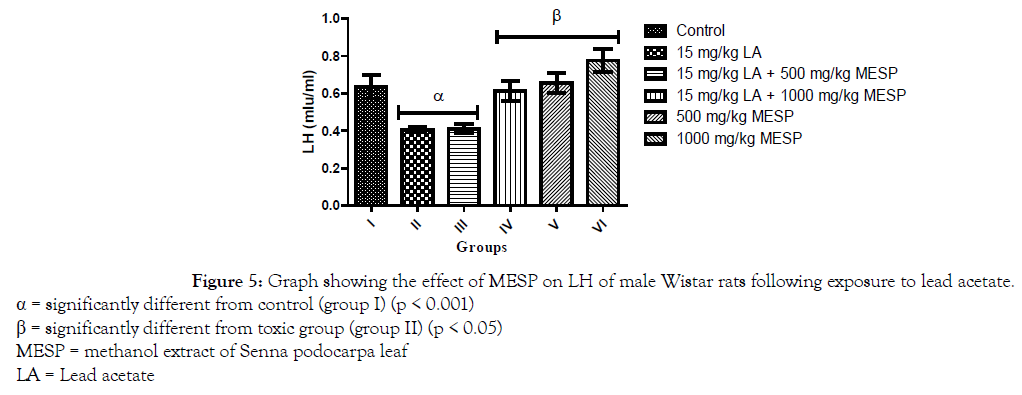
Figure 5. Graph showing the effect of MESP on LH of male Wistar rats following exposure to lead acetate.
α = significantly different from control (group I) (p < 0.001)
β = significantly different from toxic group (group II) (p < 0.05)
MESP = methanol extract of Senna podocarpa leaf
LA = Lead acetate
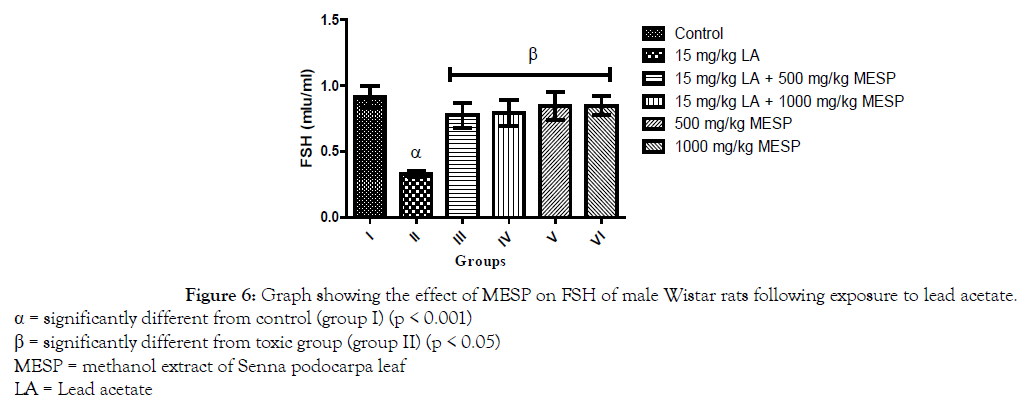
Figure 6. Graph showing the effect of MESP on FSH of male Wistar rats following exposure to lead acetate.
α = significantly different from control (group I) (p < 0.001)
β = significantly different from toxic group (group II) (p < 0.05)
MESP = methanol extract of Senna podocarpa leaf
LA = Lead acetate
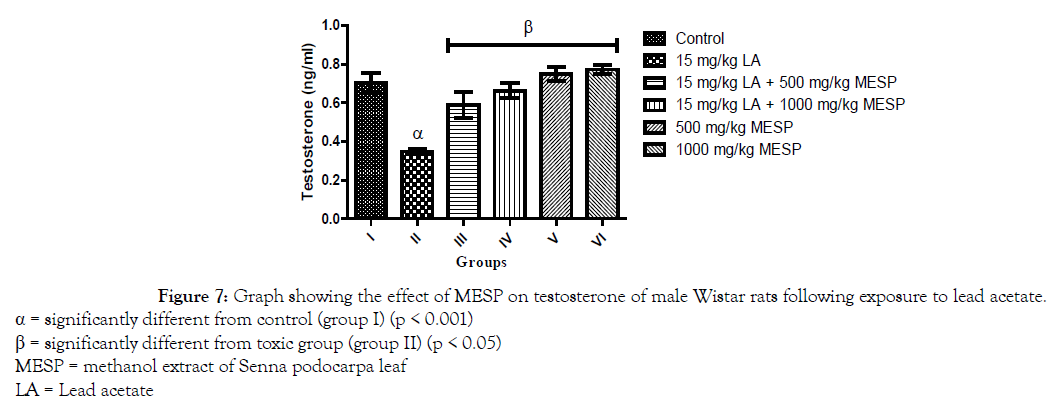
Figure 7. Graph showing the effect of MESP on testosterone of male Wistar rats following exposure to lead acetate.
α = significantly different from control (group I) (p < 0.001)
β = significantly different from toxic group (group II) (p < 0.05)
MESP = methanol extract of Senna podocarpa leaf
LA = Lead acetate
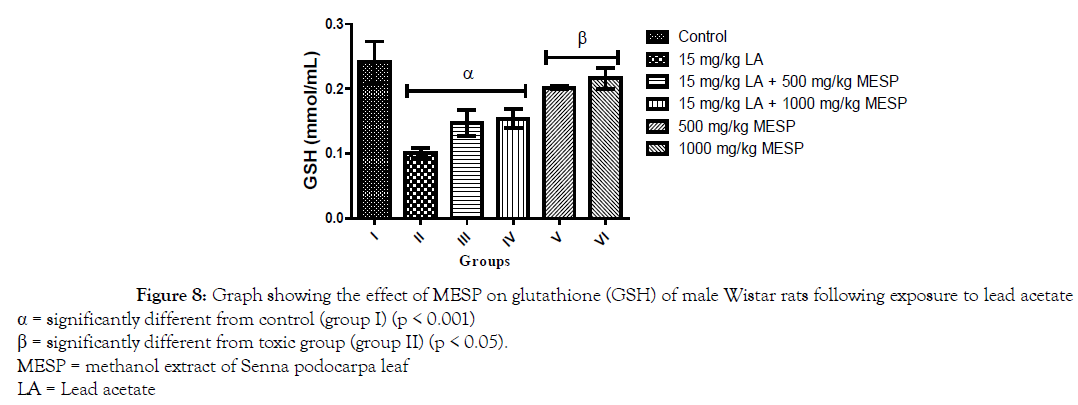
Figure 8. Graph showing the effect of MESP on glutathione (GSH) of male Wistar rats following exposure to lead acetate
α = significantly different from control (group I) (p < 0.001)
β = significantly different from toxic group (group II) (p < 0.05).
MESP = methanol extract of Senna podocarpa leaf
LA = Lead acetate
Testicular and Epididymal Histology
The histological observations from the light micrographs of control group (I) showed an apparent normal testicular histoarchitecture; the seminiferous tubules appear normal in shape (well rounded) with thin basement membrane, spermatogenic cells are well arranged showing different layers of maturation. The interstitial space appears regular in shape with its Leydig cells scattered as clusters within it. Group II showed a distorted histoarchitecture of the seminiferous tubules; seminiferous tubules appear distended with severe germ cell depletion (yellow arrow) and its lumen clogged with eroded cellular fragments and remains (blue arrow). Interstitial spaces appear disintegrated with scanty interstitial cells (red arrow). Groups III, IV, V and VI show normal histoarchitecture when compared to the control (Figure 9). Histological observation of the epididymis of the control group (I) showed normal histo-architecture of the epididymis with numerous sperms within the tubules (red arrow). The epithelial cells appear distinct and are well arranged (green arrow) leaving a regular space within the interstitium (black head arrow). Group II revealed distorted histoarchitecture of the epididymis characterized by widely spaced interstitium (blue arrow). Contents within the epididymis appear scanty and dispersed. The interstitium appears mildly eroded (black arrow). The epididymis of rats in group III showed numerous sperms within the tubules (red arrow) however, their interstitium is indistinct in appearance (yellow arrow). The epididymal histology of groups IV, V and VI rats appear apparently normal when compared with the control (Figure 10).
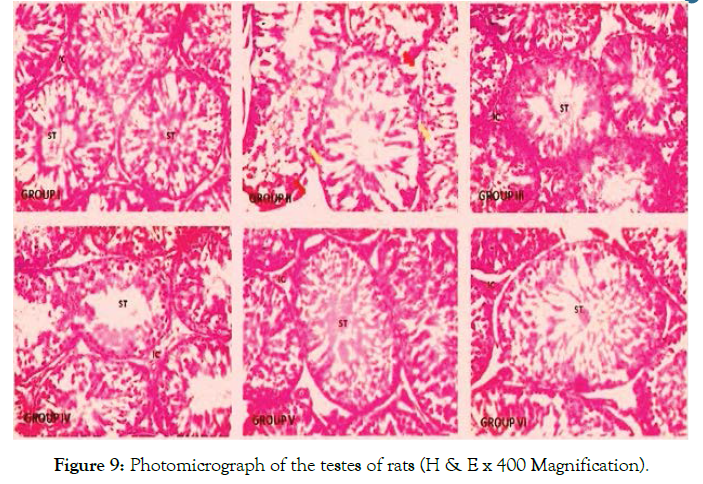
Figure 9. Photomicrograph of the testes of rats (H & E x 400 Magnification).
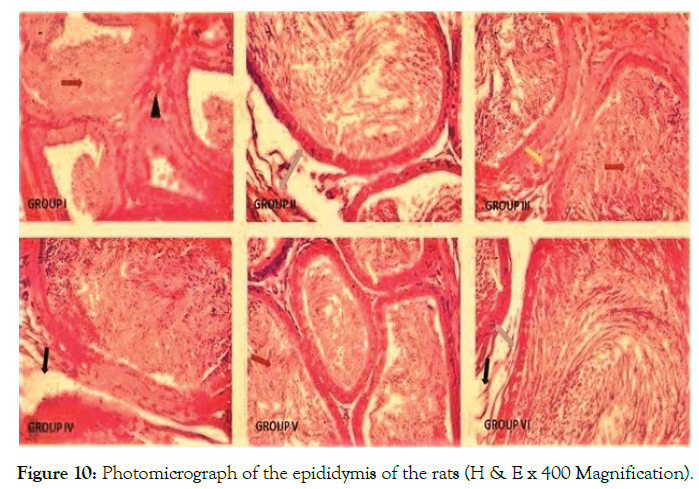
Figure 10. Photomicrograph of the epididymis of the rats (H & E x 400 Magnification).
In view of the varying reports about the continuous exposure to lead and its toxic effect on the reproductive system, this study investigated the possible ameliorative effects of the methanol extract of Senna podocarpa leaf (MESP) on lead acetate-induced reproductive toxicity using the male Wistar rats as the experimental model of choice. Analysis of body mass provides important information on the toxicity of a compound and its health implications [29]. This study reported a significant decline in body weights of rats in the lead-exposed groups when compared with the control. A similar finding was reported by Ayoka et al [4] and Daku et al [30] in their studies on lead. The rats manifested signs of weakness during the period of lead administration. The observed weight loss might be attributed to impaired metabolism of essential nutrients and intestinal malabsorption. The appreciable weight gain observed in rats administered with MESP only could mean that MESP administration caused weight gain in the rat probably by promoting a healthy appetite. The precise mechanism by which MESP achieved this result can also be further investigated. Phytochemical analysis of the plant extract by Ogunkunle and Ladejobi [31] revealed that it is rich in steroids.
The testis is a very important organ in the male reproductive system and it performs two main functions; synthesis of steroid hormones and production of sperms. The epididymis, an extension of seminiferous tubules of the testis assists in storage, transportation and also nurturing of sperm to full maturity and motility. Analysis of their absolute and relative weights may be used to evaluate the risks from adverse effects on male reproductive system owing to its high and positive correlation to sperm production [32]. In this present study it was observed that there was a significant decrease in the relative epididymal weight of the lead acetate only treated group when compared with the control. This is similar to observations of previous researchers who reported weight loss in the testis and epididymis of rats following lead exposure [2,3]. The decrease in relative testicular weight observed, though not significant might be as a result of the decrease in the number of germ cells, since the differentiated spermatogenic cell mass is the principal determinant of testicular weight [33]. On the contrary, an increase in relative reproductive organ weight was recorded when MESP-treated groups was compared to group II. The observed result might be attributed to the alkaloids and tannins found in the extract. Alkaloids have been shown to have analgesic, adaptogenic and anti-inflammatory activities which probably helped the animal to develop endurance and resistance against stress and diseases, while tannins hasten the wound healing [4].
An assessment of semen quality and quantity is an essential and widely adopted technique in diagnosis of infertility in the male subjects. An assessment of sperm count, motility, morphology and viability can reflect the reproductive function in male animals and humans. In this study, semen analysis of animals in the group administered lead showed a decrease in sperm motility, sperm viability and count while it increased the number of abnormal sperms (sperm morphology) when compared with the control group. This is similar to earlier reports on the effects of lead on sperm parameters [34,35]. Lead acetate-induced reduction in sperm count is an indication that reproductive function was impaired. The decrease in sperm motility observed in this present study may either be functional or structural or even both. A significant decrease in sperm motility and sperm viability may occur if chemical agents are able to pass through the blood-testis barrier. This compromises the reproductive and protective functions of the Sertoli cells. The decrease in sperm motility observed in our study may also be due to the ability of lead acetate to permeate the epididymis and affect the proper maturation of the sperms.
A reduction in glutathione activity (a master body antioxidant necessary to alleviate the damaging effects of free radicals) which was observed in animals exposed to lead acetate in this present study could be responsible for the degenerative effects observed the reproductive system of the rats as a whole. Reactive oxygen species can cause abnormal sperm function due to lipid peroxidation of polyunsaturated fatty acids found in the head and mid-piece of the spermatozoa thereby altering sperm morphology and causing a decrease in the motility and viability of the sperm cells. Since Deoxy-ribonucleic is also present within the head of the sperm, therefore damage to the membrane makes them vulnerable to damage. Reactive oxygen species may attack integrity of Deoxyribonucleic in the sperm nucleus [35]. Deoxy-ribonucleic acid damage caused by excessive reactive oxygen species may accelerate germ cell apoptosis, subsequent hypo-spermatogenesis and a fall in intercellular adenosine triphosphate level. This can lead to a reduction in sperm count and motility which can be associated with male infertility as observed in this current study.
It is of interest to note that in this present study, the degenerative effects observed with lead acetate administration were partially reversed when MESP was administered to the treated rats. The proposed mechanism of action of MESP in ameliorating the lead acetate-induced toxicity especially in the sperm parameters, could be alluded to its antioxidant and membrane-stabilizing activities which were made manifest by phytochemicals such as tannins, alkaloids and flavonoids found in it [36].
Spermatogenesis occurs in the presence of testosterone released by Leydig cells and it is maintained by Sertoli cells which are acted upon by follicle stimulating hormone (FSH). Luteinizing hormone (LH) secreted by the anterior pituitary gland is essential for the release of testosterone. A significant decrease in the level of serum testosterone, LH and FSH was recorded in this present study in the group administered lead acetate only. The decrease in testosterone level seen may be caused by a reduction of the secretory activity of the testes, and changes in peripheral metabolism. A relationship between blood lead levels and reproductive hormone level has also been reported [37]. Production and secretion of testosterone from the Leydig cells is under the control of luteinizing hormone. Therefore, a decrease in LH secretion will lead to a decline in testosterone secretion which will further affect the process of spermatogenesis. It can therefore be suggested that the mechanism of action by which lead acetate acts on the male reproductive system is probably by affecting the reproductive hormonal axis and thus altering the hormonal control on spermatogenesis [38]. Furthermore, this could also be as a result of a direct inhibitory activity of lead acetate on androgen production in Leydig cells [39] or a defect in LH regulation at the pituitary level [40,41] as observed by a reduction in testosterone and LH levels in this present study. However, administration of MESP increased the hormonal level of testosterone and LH as observed in this study which may imply that the extract improved spermatogenesis. This is manifested in the increased sperm count observed in the MESP treated groups when compared with the lead acetate treated group. Histological investigations revealed damages in the histo-architecture of the testis and epididymis of lead acetate treated animals. However, regenerative changes were observed in the testicular and epididymal histo-architecture of groups that were administered MESP. This might be attributed to the activity of some phytochemicals present in the plant such as tannins, saponins, terpenoids and flavonoids. These phytochemicals have been shown to possess wound-healing effects, anti-inflammatory activity and they also serve as defense chemicals [30].
Taken together, the results from this present study showed that administration of the methanol extract of Senna podocarpa leaf could ameliorate the toxic effects of lead acetate treatment observed in the epididymis and testis of the rats and also in their reproductive hormone concentration. This was evidenced by the reversal of these degenerative and toxic effects especially when compared to the group administered lead acetate only. In view of this, it is therefore suggested that studies to elucidate and isolate the active ingredient(s) present in this extract should be carried out, as this can provide a clue to the discovery of a potential profertility agent.
Citation: Akinsomisoye O.S, Akomolafe R.O, Abatan J.A, Akano O. P, Odukoya S.O.A, Akintayo C.O (2021) Effects of Methanol Extract of Senna Podocarpa leaf on the Reproductive Parameters of Male Wistar Rats Following lead Acetate Toxicity, Reproductive Sys Sexual Disord.10:257. doi: 10.35248/2161-038X.1000257.
Received: 17-Mar-2021 Accepted: 31-Mar-2021 Published: 07-Apr-2021 , DOI: 10.35248/2161-038X.21.10.257
Copyright: © 2021 Akinsomisoye O.S et al. this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.