Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Research Article - (2023)Volume 12, Issue 6
Objective and background: We aim to determine how early intervention of an MOA for dentofacial anomalies improves qualitative scores of Sleep Disordered Breathing (SDB) in school-aged children ages 5-12.
Methods: A review of historical secondary data was conducted from 1001 Sleep, Breathing and Habit Questionnaire of children ages 5-12 diagnosed with SDB in treatment with an MOA. A total of 44 sets of questionnaire scores met the inclusion criteria for the retrospective review. Eleven SDB symptom scores were reviewed at three endpoints: Initial, 2-6 months (SG1), and 7+ months (SG2).
Results: A total of 44 patient-directed sleep and breathing questionnaires from children ages 5 to 12 (female 55% (n=24), male 45% (n=20), mean age 7.54 years, ± 1.89) in treatment with an MOA for dentofacial anomalies reporting symptoms of SDB were included. Among the cohort, 90% (27 of 30) showed a statistically significant reduction of overall symptoms scored as resolved or improved from the initial visit to the 7+ months endpoint (M=15.29 months, SD ± 4.18); 71.4% (25 of 35) of the cohort showed a statistically significant reduction of overall symptoms within 2-6 months (M=4.22 months, SD ± 1.64).
Conclusion: The improvement in 4.22 months shows MOA as an effective treatment for early intervention in treating the dual pathologies of dentofacial anomalies and SDB in school-aged children ages 5-12.
Sleep; Sleep Disordered Breathing (SDB); Nasal obstruction; Obstructive Sleep Apnea (OSA); Pediatrics; Repositioning device; Oral appliance; Monoblock; Children
Sleep Disordered Breathing (SDB) is increasingly common and defined as a group of disorders characterized by abnormal breathing patterns such as hypopneas or apneas, or inadequate ventilation during sleep that disrupts the sleep pattern leading to Obstructive Sleep Apnea (OSA) [1]. The prevalence of breathing disorders during sleep in school-aged children 5-12 years of age is between 1 and 4% with habitual snoring prevalence at about 7.45% [2]. In a retrospective analysis of data reported by parents, Lumeng, et al. [3] found similar prevalence estimations reported by parents: Parent-reported "always" snoring, 1.5 to 6%; parentreported apneic events during sleep, 0.2 to 4%; SDB by varying constellations of parent-reported symptoms on questionnaire, 4 to 11%; OSA diagnosed by varying criteria on diagnostic studies, 1 to 4%. Overall prevalence of parent-reported snoring by any definition in meta-analysis was 7.45% (95% confidence interval, 5.75-9.61) [3].
Other studies suggest OSA may be a far more common complaint, with upper airway resistance and snoring reported as high as 27% [4]. Furthermore, undiagnosed "silent" Obstructive Sleep Apnea (OSA) occurring in Dentofacial Deformities (DFD) patients with primary mandibular deficiency and short face DFDs (p<0.001 and p=0.001, respectively) [5].
Guilleminault, et al. [6], first reported the diagnosis of OSA in children 5 to 14 years of age. Theories and hypotheses explaining the connection between SDB, OSA, oral-facial growth, dentofacial anomalies, and when they occur have been debated and studied over the past decade [7-9]. Huang, et al.[10], reviewed evidence hypothesizing the connection of OSA and oral-facial growth. Their findings showed the association between pediatric sleep issues and abnormal breathing affecting facial hypotonia and concluded abnormal oral-facial anatomy that must be treated in order for the resolution of OSA [10]. Dentofacial anomalies are common craniofacial abnormalities resulting as a substantial risk for sleep deficiencies, Breathing Disorders (BD) and Obstructive Sleep Apnea (OSA) [7-10]. The developing oral-facial anatomy has an impact on the teeth and alveolar processes of the face where disease and systemic conditions manifest as soon as the embryonic phases [11]. Previous studies found a causal relationship between upper respiratory obstruction and dentofacial abnormalities related to genetic or structural issues resulting in maxillary hypoplasia, mandibular condylar hypoplasia, retrognathism, or narrow palatal arch [12]. Within the pediatric population, it has been hypothesized an oral appliance in early intervention may address OSA and dentofacial anomalies prior to more aggressive interventions; however, the durability and adherence of a tooth positioner should be considered [13,14]. It has been hypothesized Monobloc Oral Appliances (MOA) have a positive effect in reducing symptoms of breathing disorders at sleep in school-aged children. de Rutier, et al. [15], concluded the efficacy of a Sleep Position Trainer (SPT) comparable to an Oral Appliance (OAT) with a relatively high adherence [15,16]. Therapy for OSA has been discussed. Capan, et al. [8], initially found MOAs to be an effective treatment option for children with retrognathia and OSA as evidenced by a reduction in the Apnea Hypopnea Index (AHI). Another study by Capan, et al. [12], and Issaccson, et al. [17], found MOAs may also improve the behavior in children with snoring symptoms and skeletal class II malocclusions. Corrective action using a monobloc appliance shows comparable corrective action to a bibloc appliance in treating OSA; however, the monobloc device reduced the ODI at greater level than a bibloc with a lower cost of treatment after one year by 17% and with greater patient compliance [17]. Breathing disorders during sleep begin at an early age. Changes in the pediatric airway resulting in the paradigm of Sleep Disordered Breathing (SDB) increase the risk of adulthood comorbidities and disease states physically and psychologically [18]. Such comorbidities from unresolved or unimproved SDB symptoms can lead to hypertension, cor pulmonale, bronchopulmonary dysplasia, sickle cell disease, obesity, insulin resistance, failure to thrive (malnutrition), Attention-Deficit/Hyperactivity Disorder (ADHD), and major depressive disorder [18]. Furthermore, pediatric Sleep Disordered Breathing (SDB) is a continuum of symptoms ranging from snoring to Obstructive Sleep Apnea (OSA) that can differ from adult OSA symptoms [19,20]. However, research shows symptoms are a result of downstream problems in natural nasal breathing The multisymptomatic presence of SDB can occur with 2 symptoms or as many as 11, as found on various subjective, patient directed data questionnaires, including the one used in this study [21]. The role of airway dentistry assessing nasal function and for OSA symptoms has come to the forefront in order to collaborate with the medical community addressing SDB, specifically with oral devices such as an MOA. For the purposes of this study, the eleven symptoms for analysis commonly seen in pediatric OSA were snoring, snore interruption, labored breathing, mouth breathing, restlessness, teeth grinding, sleep talking, sweating, waking up, bed wetting, and daytime sleepiness.
Data sampling
WCG IRB’s IRB Affairs Department reviewed the study under the Common Rule and applicable guidance determining the study is exempt with a waiver of consent under 45 CFR § 46.104(d)(4). Informed consent was obtained by the provider prior to treatment. The sample of forty-four questionnaire scores were retrieved from a secondary database consisting of data derived from multiple centers from December 2018 to July 2020. A cluster sampling technique was used according to utility of an MOA and the secondary data scores from the Sleep, Breathing and Habit Questionnaire collected by the Airway Intelligence Service (AIS) at three endpoints (Appendix). Inclusion criteria included the questionnaire scores from children between the ages 5 and 12 with symptoms of breathing disorders while asleep and in treatment with an MOA oral appliance. Exclusion criteria were questionnaire scores of children below the age of five and older than twelve, anyone involved in an Investigational Review Board (IRB) clinical trial, patients with known genetic conditions affecting the airway such as Down syndrome, and subjects not in MOA oral appliance treatment. The primary data endpoints were the scores from 11 symptoms recorded on the questionnaire at the initial visit, and follow-up visits at 2-6 months, and 7+ months for a total of 3 endpoints.
Questionnaire structure and procedures
The data was collected and coded as a nominal value in the data set: Gender, coded as 1 for female and 2 for male, age, length of MOA treatment, individual scores of 0 to 3 for Snoring, Snore interruption, Labored breathing, Mouth breathing, Restlessness, Teeth grinding, Sleep talking, Sweating, Waking up, Bed wetting, and Daytime sleepiness. Frequency scores were defined as 0 for No occurrence, 1 for Rarely Occurred, 2 for Occurred 2-3 times per week, and 3 for Occurred 5-7 times per week. Nominal value scoring for SDB symptoms were collected from parents at each visit: The initial visit and follow-up visits at 2-6 months (coded as SG1), and 7+ months (grouped by greater than or equal to 7) for a total of 4 endpoints.
Monobloc Oral Appliance (MOA)
An MOA is an FDA-registered, Class I orthodontic tooth positioner and orthodontic appliance designed as an upper and lower fused together to form a single unit for patients 4 years of age and up. MOAs are contraindicated in cases of non-nasal breathing patients, restricted oral tissues, and severe gag reflex issues. Manufactured with a durable polymer material, they are easily removable, with design features to guide growth and development, promote nasal breathing, decrease the symptoms of sleep disordered breathing, and reposition and retrain the tongue to sit more up and forward in the mouth. The myofunctional aspects of an MOA promote tongue pressure anteriorly with the indirect association of maxillary expansion, yet to be proven in clinical trials. The recorded data in this study is from treatment with either one or more of the appliances as part of the overall treatment regimen as determined by their treating dentist (Figure 1).
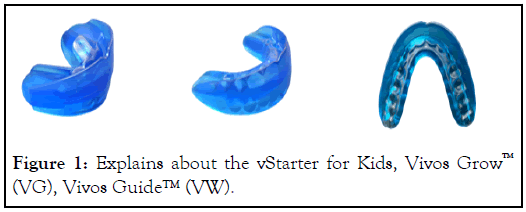
Figure 1: Explains about the vStarter for Kids, Vivos Grow™ (VG), Vivos Guide™ (VW).
Examples of MOAs: vStarter for Kids is used in patients who typically have not had their first molars erupt yet. vStarter used in patients who typically have their first molars either partially erupting or fully erupted. Vivos Grow (VG as is sometime abbreviated) used primarily in mixed dentition patients and come in 10 different sizes. Vivos Guide™ (VW) specifically designed for permanent dentition to control 2nd permanent molars
Statistical analysis
IBM SPSS Statistics software (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, version 28.0. Armonk, NY; IDM Corp.) was used to perform the statistical analysis and features and summaries of the data set. A mean comparison pairwise experimental design was used to compare SG1 and SG2 questionnaire results, setting up the following null hypothesis tested at the 0.01 significance level to test if there was a statistically significant difference between the sample groups. Prior to using pairwise statistical t-test for mean comparison, the following assumptions were considered and met:
1. Mean of both samples (initial and final results) follow a normal distribution according to the central limit theorem where the sample size is large enough and meet the assumption with SG1 and SG2 having sample sizes of 35 and 30, respectively.
After meeting the assumptions, parametric paired-samples t tests were utilized in this nonexperimental study design to evaluate the relationship between the probability and evidence of early intervention improving or resolving SDB symptoms and use of an MOA over time with the patients acting as their own control. The paired samples to test was used for evaluating the differences within paired samples at each endpoint with a pvalue< 0.01 considered statistically significant. Descriptive statistics were completed to quantitatively describe the collection of data and summarize the changes in occurrence of the eleven (11) SDB symptoms of the sample over time at each endpoint for an analysis to identify the groups of patients that had the same improvement and resolve of their symptoms. Each symptom had an occurrence score from 0-3 to measure the presence of the symptom reported by the parents or caretaker. To summarize the prevalence of each symptom at each end point, a comparative calculation was completed by utilizing a general percentage change formula for each individual symptom by subtracting the sum of the sample indicating symptom prevalence (B) from the initial endpoint (A) and dividing by the initial endpoint (A) then multiplying by 100:
Individual Symptom Prevalence Percent Change=(B-A)/A*100
Once all the individual symptom prevalence percent changes were calculated an average formula was utilized to get the average percent change across all symptoms:
Average percent change across all symptoms=Symptom Percent Change1+Symptom Percent Change2+...+Symptom Percent Changen)/n
Demographics and main clinical features
A review of 1001 records resulted in sample data from 44 Sleep, Breathing and Habit Questionnaires of children ages 5-12 (female 55% (n=24), male 45% (n=20), mean age 7.54 years, ( ± 1.89) completed at three endpoints (Tables 1 and 2).
| Patient age | ||||
|---|---|---|---|---|
| Years | Frequency | Percent | Percent of males | Percent of females |
| 5 | 8 | 18.2 | 50 | 50 |
| 6 | 7 | 15.9 | 86 | 14 |
| 7 | 3 | 6.8 | 33 | 67 |
| 8 | 15 | 34.1 | 33 | 67 |
| 9 | 5 | 11.4 | 20 | 80 |
| 10 | 3 | 6.8 | 33 | 67 |
| 11 | 2 | 4.5 | 50 | 50 |
| 12 | 1 | 2.3 | 100 | - |
Table 1: Frequency distribution (n=44).
| No occurrence | Occurs rarely | Occurs 2-4 times per week | Occurs 5-7 times per week | |
|---|---|---|---|---|
| Snoring | 25.00% | 43.20% | 18.20% | 13.60% |
| Snore interruption* | 88.10% | 7.10% | 0.00% | 4.80% |
| Labored breathing | 61.40% | 22.70% | 9.10% | 6.80% |
| Mouth breathing | 22.70% | 25.00% | 20.50% | 31.80% |
| Restlessness | 34.10% | 25.00% | 25.00% | 15.90% |
| Teeth grinding* | 39.00% | 14.60% | 17.10% | 29.30% |
| Sleep talking* | 34.90% | 41.90% | 16.30% | 7.00% |
| Sweating* | 61.00% | 24.40% | 14.60% | 0.00% |
| Waking up | 29.50% | 34.10% | 18.20% | 18.20% |
| Bed wetting | 86.40% | 6.80% | 4.50% | 2.30% |
| Daytime sleepiness* | 45.20% | 21.40% | 26.20% | 7.10% |
Note:*Missing values for snore interruption (-2), teeth grinding (-3), sleep talking (-1), sweating (-3), daytime sleepiness (-2).
Table 2: Baseline symptoms at the initial visit (n=44).
Evaluation of symptoms and occurrence
In the study group, Figure 2 shows the occurrence rates reported on the questionnaire showed improvement over time from 5-7 (score of 3) times a week and 2-4 (score of 2) times per week to rarely occurring (score of 1) and not occurring (score of 0) at the 2-6 month endpoint and 7+ months.
Figure 2: The average count of reported SDB symptoms occurred 2 to 7 times per week throughout treatment. Note: *0 Month N=44, 2-6 Month N=35, 7+Month N=30.
Two-sided paired t-tests were conducted between SG1 and SG2 to evaluate the resolution of SDB symptoms occurring often (2-7 times per week) in children using an MOA at each endpoint. The results indicated a significance in the reduction of overall symptoms occurring often from the initial visit (M=2.97, SD ± 2.31) to the second endpoint at 2-6 months (M=1.86, SD ± 1.82), with an average reduction of 1.09 symptoms (99% CI (0.09, 2.14); p<0.01) and from the initial visit (M=3.17, SD ± 2.31) to the 7+ endpoint (M=1.20, SD ± 1.63), with an average reduction of 1.97 symptoms (99 % CI (0.74, 3.19); p<0.01). The overall results indicated 54.3% (19 of 35) of SG1 saw symptoms resolved to rarely to not occurring at 2-6 months (M=4.22 months, SD ± 1.64), and 66.7% (20 of 30) of SG2 saw resolved symptoms at 7+ months (M=15.29 months, SD ± 4.18) described in Table 3.
| 2-6 Months (n=35) | 7+ Months (n=30) | |
|---|---|---|
| Number of patients who saw reduction in symptoms occurring 2-7 times a week | 19 | 20 |
| Percent of patients who saw reduction in symptoms occurring 2-7 times a week | 54.3% | 66.70% |
| p-Value | <0.01 | <0.01 |
Table 3: Percent of sample with decrease in the number of symptoms occurring from 2-7 times a week to rarely occurring to no occurrence.
Changes in symptom characteristics
SG1 showed an improvement in all symptoms with an average change of 11.1% of the symptoms being resolved (Table 4). Likewise, SG2 saw symptoms resolved by an average change of 46.4% (Tables 5). The most notable change for any symptom was seen in Mouth Breathing and Teeth Grinding which saw a 77.8% and 80% reduction, respectively, in occurrence rate at the 5-7 times a week from the initial visit to the 7+ month visit (Table 5).
| Sum of all occurrence rates | Sum of 2-7 times per week occurrence rates | Sum of 5-7 times per week occurrence rate | Sample size | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | 2-6 Month | % Change | Initial | 2-6 Month | % Change | Initial | 2-6 Month | % Change | Initial | Follow-Up | Differential | |
| Snoring | 25 | 26 | 4.00% | 8 | 5 | -37.50% | 3 | 1 | -66.70% | 34 | 34 | 0 |
| Snore interruption | 5 | 4 | -20.00% | 2 | 1 | -50.00% | 2 | 0 | -100.00% | 34 | 33 | -1 |
| Labored breathing | 15 | 13 | -13.30% | 6 | 3 | -50.00% | 2 | 1 | -50.00% | 35 | 34 | -1 |
| Mouth breathing | 26 | 26 | 0.00% | 16 | 12 | -25.00% | 9 | 3 | -66.70% | 35 | 34 | -1 |
| Restless | 24 | 18 | -25.00% | 15 | 10 | -33.30% | 6 | 2 | -66.70% | 35 | 34 | -1 |
| Teeth grinding | 18 | 16 | -11.10% | 14 | 7 | -50.00% | 8 | 2 | -75.00% | 33 | 33 | 0 |
| Sleep talk | 21 | 19 | -9.50% | 8 | 6 | -25.00% | 2 | 0 | -100.00% | 34 | 35 | 1 |
| Sweating | 14 | 8 | -42.90% | 6 | 2 | -66.70% | 0 | 1 | 100.00% | 33 | 35 | 2 |
| Waking up | 25 | 24 | -4.00% | 15 | 10 | -33.30% | 8 | 4 | -50.00% | 35 | 35 | 0 |
| Bed wetting | 5 | 4 | -20.00% | 2 | 1 | -50.00% | 1 | 1 | 0.00% | 35 | 34 | -1 |
| Day sleepiness | 15 | 18 | 20.00% | 11 | 8 | -27.30% | 2 | 0 | -100.00% | 33 | 34 | 1 |
| -11.10% | -40.70% | -52.30% | ||||||||||
Table 4: Changes in symptom characteristics of SG1(n = 35).
| Sum of all occurrence rates | Sum of 2-7 times per week occurrence rates | Sum of 5-7 times per week occurrence rate | Sample size | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | 7+ Month | % Change | Initial | 7+ Month | % Change | Initial | 7+ Month | % Change | Initial | Follow-Up | Differential | |
| Snoring | 24 | 14 | -41.70% | 11 | 4 | -63.60% | 3 | 1 | -66.70% | 30 | 30 | 0 |
| Snore interruption | 2 | 0 | -100.00% | 0 | 0 | 0.00% | 0 | 0 | 0.00% | 28 | 30 | 2 |
| Labored breathing | 10 | 1 | -90.00% | 5 | 0 | -100.00% | 2 | 0 | -100.00% | 30 | 30 | 0 |
| Mouth breathing | 23 | 18 | -21.70% | 17 | 8 | -52.90% | 9 | 2 | -77.80% | 30 | 30 | 0 |
| Restless | 20 | 11 | -45.00% | 13 | 5 | -61.50% | 5 | 2 | -60.00% | 30 | 30 | 0 |
| teeth grinding | 19 | 12 | -36.80% | 15 | 5 | -66.70% | 10 | 2 | -80.00% | 28 | 30 | 2 |
| Sleep talk | 19 | 10 | -47.40% | 7 | 3 | -57.10% | 2 | 1 | -50.00% | 30 | 30 | 0 |
| Sweating | 12 | 9 | -25.00% | 4 | 0 | -100.00% | 0 | 0 | 0.00% | 28 | 30 | 2 |
| Waking up | 22 | 15 | -31.80% | 9 | 6 | -33.30% | 3 | 2 | -33.30% | 30 | 30 | 0 |
| Bed wetting | 2 | 1 | -50.00% | 1 | 1 | 0.00% | 0 | 0 | 0.00% | 30 | 30 | 0 |
| Day sleepiness | 19 | 15 | -21.10% | 13 | 4 | -69.20% | 3 | 0 | -100.00% | 30 | 29 | -1 |
| -46.40% | -55.00% | -51.60% | ||||||||||
Table 5: Changes in symptom characteristics of SG2 (n=30).
Overall score evaluation
Analysis of the results for individual patient scores and the average total score of each of their symptoms within each endpoint group indicated a significance in the mean of overall symptoms resolving or improving from the initial visit (M=0.91, SD ± 0.54) to the second endpoint at 2-6 months (M=0.68, SD± 0.37), with an average reduction in their overall average score of 0.24 (99% CI (0.04, 0.43); p<0.01), and the initial visit (M=0.94, SD ± 0.54) to the 7+ endpoint (M=0.46, SD ± 0.37, with an average reduction in their overall average score of 0.48 (99% CI (0.25,0.71); p<0.01). The mean overall improvement of both SG1 and SG2 correlates to the findings that 71.4% (25 of 35) of patients will have improvement or resolution of all SDB symptoms within 2-6 months (M=4.22 months, SD ± 1.64) and 90% (27 of 30) after 7+ months (M=15.29 months, SD ± 4.18) of MOA treatment therefore, there is compelling evidence that on average, MOA leads to SDB symptom improvement and resolve (Table 6).
| 2-6 Months (n=35) | 7+ Months (n=30) | |
|---|---|---|
| Number of patients with decreased overall SDB score | 25 | 27 |
| Percent of patients with decreased overall SDB score | 71.4% | 90.0% |
| p-value | <0.01 | <0.01 |
Table 6: Percent of sample with decreased overall SDB score.
SDB is defined as a group of disorders characterized by abnormal breathing patterns such as hypopneas or apneas, or inadequate ventilation during sleep that disrupts the sleep pattern [22]. The main findings of the study showed that there is compelling evidence that MOA therapy leads to SDB symptom improvement and resolve as indicated by statistical measures evaluating symptoms reducing in severity from 2-7 times a week to rarely or not occurring at all, along with overall average score reduction from endpoint to endpoint. When looking at SG1, the findings showed 54.3% of patients will see a decrease in their most severe symptoms, and 71.4% will see overall improvement as soon as 2.58 months with an average of 4.22 months. During this time there were noticeable changes in 25%or more with common and recognizable symptoms such as mouth breathing, snoring, waking up at night, and teeth grinding that were reduced to rarely occurring or not occurring at all. As patients progressed in treatment, the percentage of patients seeing a decrease in their severe symptoms and overall score improvement elevated to 66.7% and 90% as results indicated by the SG2 with an average of 15.26 months of treatment.
SG2 displayed descriptive statistical characteristics of symptoms being alleviated to no rate of occurrence on average of 46.4% according to Table 5 sum of all occurrence rates (Sum of all patients who indicated a score of 1, 2 or 3). Symptoms such as Mouth Breathing and Daytime Sleepiness where the prevalence was high initially, particularly in the more severe range of occurrence rates of 2 to 7 times a week only saw 21.7% and 21.1% reduction in the rate of no occurrence meaning these symptoms were still occurring rarely. However, they saw dramatic reduction in occurring 2-7 times a week by 52.9% and 69.2% respectively. This is impactful due to the daytime sleepiness effects on daily activities causing more sports injuries, mood, behavioral and concentration problems along with the clinical manifestations of mouth breathing that occur in children with OSA, and as a notable symptom seen by parents [23-25]. The novel MOA was efficacious in an overall improvement of all SDB symptoms from the study sample from the initial visit to the 7+ month endpoint correlates to the findings that 90% of patients will have improvement of resolution of all SDB symptoms (p=<0.01). These findings are significant in sleep health, cognitive function, quality of life, and childhood development. The findings allow healthcare providers to better educate the patient and their families on what to expect with the therapy. Despite the number of symptoms presenting for SDB diagnosis and treatment, the treatment of SDB will often start with a Tonsillectomy and Adenoidectomy (T & A) with a followup polysomnography done at 6-8 weeks post-operatively [26,27]. Huang, et al. [28], noted the surgical outcomes of prepubescent children having a T & A initially thought to be cured of OSA were found to have OSA as teens. Likewise, children with at least one oropharyngeal abnormality in post T & A showed clinical symptoms of OSA and negative PSG tests at the 3 months post-operative visit [29]. In a study by Guilleminault, et al. [30], found the incomplete postsurgical results from T&A may preclude the fact that not all children will need a T&A, and the enlarged lymphoid tissue may be a consequence not a cause to the spectrum of breathing disorders [29]. The long-term effects of T&As have not been considered. In a longitudinal study of 1.2 million kids, Byars, et al. [31], found the removal of adenoids or tonsils in childhood was associated with significantly increased relative risks of later respiratory, allergic, and infectious diseases as long term as 30 years. Conclusively, an increase in long-term absolute disease risks were considerably larger than changes in risk for the disorders these surgeries aim to treat, and the risks of these surgeries warrant careful consideration [30]. In a retrospective study by Lee, et al. [32], concluded the need to assess mouth breathing prior to T&A due to potential of residual SDB. Based on the findings of improved breathing and previous studies that found breathing disorders after surgery, the MOA deserves consideration prior to surgery in children [32]. The results of mouth breathing can originate at birth with Dentofacial Deformities (DFD) and Craniofacial Anomalies (CFA) noted among the most common birth defects affecting many children ranging from tooth size, shape, and structure abnormalities as a result from disturbances during the morpho-differentiation stage of development [33-38]. Altug, et al. [39], concluded, “It is important to treat these anomalies because they can create disturbances in maxillary and mandibular dental arch lengths and occlusions; these problems might complicate orthodontic treatment planning”. Huang, et al. [28], discussed the aspect of orthodontia and high arches as it relates to nasal flow limitations and growth. Founded on the conclusions of Harvold, et al. [40], breathing disruptions induce fundamental changes in establishing nasal breathing and eliminating mouth breathing may well be the only valid endpoint for treating pediatric OSA and are easily conformable to treatment. Further research by Xie, et al. [41], found the impact of craniofacial manipulation and the therapeutic mechanism of action with Rapid Maxillary Expansion (RME) included a way to increase upper airway volume, reduce nasal resistance, and change tongue posture that affect the craniofacial morphology.
Ear Nose and Throat surgeons (ENT) suggest sufficient nasal airflow with surgical approaches; however, difficulties with airflow in children with a deep palatal vault severe and maxillary width deficiency remain due to the vault of the palate occurring simultaneously as the floor of the nasal passages [42,43]. With a sensitivity of 91% and specificity of 96% for proper patient identification and stratification prior to OSA surgery, the thought is to measure nasal function and resistance with 4-phase rhinomanometry [44,45]. Nasal function testing would also identify MOA therapy as an appropriate treatment option in children as young as three years old [46,47]. In the literature review, early intervention using MOA treatment was found to reduce the AHI and OSA symptoms in children which are important in preventing sequelae of health events as they mature into adults [48]. Furthermore, clinical guidelines, practice parameters, and algorithms for oral appliance therapy have not been updated by the American Academy of Sleep Medicine and American Academy of Dental Sleep Medicine since 2015 [49]. The guidelines for OSA suggest Continuous Positive Airway Pressure (CPAP) as the first line of therapy and oral appliances as secondary, or in place of “nothing” with a collaboration between sleep physicians and dentists; however, the guidelines do not recognize the importance of nasal breathing, early intervention, nor nasal obstruction and the assessments of nasal function for SDB [50]. Additionally, research in the literature review showed a favorable opinion towards customized, titratable oral appliances over non-customized oral appliances [48]. This may prohibit the MOA as a universally recognizable and beneficial treatment option for early intervention prior to CPAP or surgery in children [51]. In line with the clinical policy bulletins from third party payors that recognize CPAP and surgery as treatment options for children prior to MOA treatments, MOAs are important considerations in early treatment and intervention for children before OSA comorbidities appear in adulthood. Studies have recently shown an evidence-based medicine approach using MOA may, to some degree, increase compliance [52]. This realworld, multicenter non-interventional study (NIS) describes the treatment patterns, effectiveness, and safety of current treatment using an MOA. Furthermore, it allows for an alternative to early intervention and care to resolve and or improve multiple SDB symptoms. By resolving or improving the symptoms of SDB with dentofacial anomalies, the quality of life, development, and sleep habits for kids can be improved while ascertaining the prevention of potential comorbidities as they mature [53,54]. The data analysis of outcomes at all endpoints allows healthcare providers to better monitor the progress of treatment and educate families and patients on the expectations of the MOA therapy with dentofacial anomalies [54-57]. The findings of the study also support standardization and development of educational tools for practitioners to prepare for the treatment with MOA. The regimens in treating children with SDB in the United States and globally aim to put this study findings into context of the current treatment landscape excluding investigational therapies. The study results may be used to support future health technology assessment submissions and publications. This retrospective study is the first of its kind that examines multiple SDB symptoms and the use of an oral device as an alternative to the current modalities of treatment, surgery, and therapy.
The presence of dentofacial anomalies and SDB symptoms are considered markers of poor nasal breathing affecting craniofacial development in children ages 5-12, and younger. In this retrospective study analyzing 11 symptoms of SDB from questionnaire scores of 44 children ages 5 to 12 in monobloc MOA treatment, we found immediate improvement of SDB symptoms occurred from initial visit to the endpoint at 2 to 3 months. We also found a plateau of resolving or improvement of symptoms between the 2 to 3 months endpoint and the 4-6 months endpoint, but most profoundly, there is a high probability that 90% of children in MOA therapy will have SDB symptoms resolved or improved at the 7+ month endpoint. The most commonly observed symptoms of SDB such as snoring, mouth breathing, and bedwetting were significantly improved at the 2 to 3 month endpoint.
Conclusively, an MOA with early intervention specifically between the ages of 5 to 8 has a statistically signficant impact on resolving and reducing sleep disordered breathing symptoms ultimately improving physiological and emotional health and development of children. Additionally, the data analysis of outcomes at three endpoints allows healthcare providers to better monitor the progress of treatment and educate families and patients on the expectations of the MOA therapy.
Limitations of the study included the data collection of scores from questionaries based on parental reports; therefore, the subjective scoring may have an emotional component and lack precision. Validity of parental ratings and the ratings on the questionnaire may be based on how they felt their child was sleeping and not as a witness watching their child sleep every night. There may be a bias by the parent as parents of children with sleep difficulties and health conditions may be sensitive to the topic. Second, the study sample size at each endpoint was small and asymmetrical at each endpoint requiring a valid nonparametric statistical test to find normality and strong evidence that MOA treatment leads to SDB symptom improvement and resolve. A summary of changes showing the improvement of symptoms was done through descriptive statistics and mathematical calculations over time as each endpoint lacked equal variances for time series testing and cross correlations. Third, the sleep disorder questionnaires can identify SDB symptoms, but they do not diagnose OSA. Talk about compliance as that information was not consistently recorded or not recorded at all. Fourth, the sleep disorder questionnaires can identify SDB symptoms, but they do not diagnose OSA. Fifth, compliance was qualified by the parent and verbal confirmation of use, but objective measurement tools need to be developed for future studies. Lastly, sleep studies and nasal function studies to assess nasal breathing were not completed in all patients because of cost, access, or young age.
We are grateful to Marc Adelberg DDS, Stacey Kreuz DDS and Dimitrios Zikos, Ph.D. MSc, BSN for their valuable contributions.
Karen Davidson. No conflicts. Toshi Hart is a speaker, receives an honorarium for courses, and is a very small shareholder in Vivos. Corby Dixon is a supervisor for Airway Intelligence at Vivos and a very small shareholder in Vivos. Zach Wilde. No conflicts. There was no financial support for the study nor bias for this retrospective data as it was stored prior to any interest in Vivos Therapeutics. The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with the article can be viewed.
This article does not contain any studies with human participants or animals performed by any of the authors.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
Citation: Davidson KP, Dixon C, Wilde Z, Hart T (2023) Effectiveness of Early Intervention with a Monobloc Oral Appliance in Reducing Symptoms of Breathing Disorders at Sleep in Children with Dentofacial Anomalies Ages 5-12: A Retrospective, Multicenter Analysis. J Sleep Disord Ther. 12:446.
Received: 29-May-2023, Manuscript No. JSDT-23-25196; Editor assigned: 31-May-2023, Pre QC No. JSDT-23-25196(PQ); Reviewed: 14-Jun-2023, QC No. JSDT-23-25196; Revised: 23-Jun-2023, Manuscript No. JSDT-23-25196(R); Published: 30-Jun-2023 , DOI: 10.35248/2167-0277.23.12.446
Copyright: © 2023 Davidson KP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.